
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM
For the fiscal year ended
or
For the transition period from _________ to _________
Commission file number:

(Exact name of registrant as specified in its charter)
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
(Address of principal executive offices including zip code) |
( (Registrant’s telephone number, including area code) |
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
|
Title of Each Class |
Trading Symbol(s) |
Name of Each Exchange on Which Registered |
SECURITIES REGISTERED PURSUANT TO SECTION 12(G) OF THE ACT: NONE
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Accelerated Filer ☐ | ||
| Non-accelerated Filer ☐ | Smaller reporting company | |
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting firm that prepared or issued its audit report.
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐
The aggregate market value of the registrant’s
common stock held by non-affiliates of the registrant as of February 26, 2021, the last business day of the registrant’s most recently
completed second fiscal quarter, based upon the closing price of the common stock as reported by The Nasdaq Global Capital Market on such
date, was approximately $
As of October 25, 2021, shares of the registrant’s common stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Certain portions of the registrant’s definitive proxy statement to be delivered to its shareholders in connection with the registrant’s 2022 Annual Meeting of Shareholders are incorporated by reference into Part III of this Annual Report on Form 10-K. Such definitive proxy statement will be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
Simulations Plus, Inc.
FORM 10-K
For the Fiscal Year Ended August 31, 2021
Table of Contents
| i |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, concerning our business, operations, and financial performance and condition, as well as our plans, objectives, and expectations for our business operations and financial performance and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about:
| · | the continued growth in demand for our software products and consulting services in the pharmaceutical drug development industry; |
| · | our sales, marketing, and distribution prospects; |
| · | the continuing productivity and effectiveness of our commercial infrastructure and worldwide salesforce; |
| · | our financial performance; |
| · | our expectations regarding the worldwide market for modeling and simulation services; |
| · | the continued competitive positioning of our software products and services; |
| · | our expectations regarding the potential market size and the continuing expansion of modeling and simulation usage in the drug development sector; |
| · | the scope of protection we are able to maintain for intellectual property rights covering our software products; | ||
| · | the modeling and simulation software and services market; | ||
| · | the continued expectations of growth for pharmaceutical drug development research and development expenditures; |
| · | our ability to grow our consulting staff to meet demand and retain scientific employees to perform consulting services for our customers; |
| · | the continuing support of the use of modeling and simulation by regulatory authorities like the U.S. Food and Drug Administration (FDA); |
| · | the implementation of our business model and strategic plans for our business and technology; |
| · | our expectations regarding the effects of the COVID-19 pandemic on our business and our clients’; and |
| · | developments and projections relating to our competitors and our industry. |
These forward-looking statements are based on management’s beliefs and assumptions, as well as management’s current plans, expectations, estimates, forecasts, and projections about our business and the industry in which we operate and are not guarantees of future performance or development. These forward-looking statements involve known and unknown risks, uncertainties, and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this Annual Report on Form 10-K may turn out to be inaccurate and actual results or events could differ materially from the plans, expectations, estimates, forecasts, and projections disclosed in the forward-looking statements that we make. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under “Risk Factors” and elsewhere in this Annual Report on Form 10-K. Investors are urged to consider these factors carefully in evaluating the forward-looking statements. These forward-looking statements speak only as of the date of this Annual Report on Form 10-K. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
| 1 |
PART I
ITEM 1 –BUSINESS
As used in this Annual Report Form 10-K, each of the terms “we,” “us,” “our,” the “Company” and “Simulations Plus” refers to Simulations Plus, Inc. and its wholly owned subsidiaries (both current and previous, as applicable) Cognigen Corporation, of Buffalo, New York; DILIsym Services, Inc. of Research Triangle Park, North Carolina; and Lixoft of Paris, France, unless otherwise stated or the context otherwise requires.
OVERVIEW
Simulations Plus, Inc., incorporated in 1996, is a premier developer of modeling and simulation software for drug discovery and development, including the prediction of properties of molecules utilizing both artificial intelligence (“AI”) as well as machine-based technology. We also provide consulting services ranging from early drug discovery through preclinical and clinical trial development to regulatory submissions in support of product approval. Our software and consulting services are provided to major pharmaceutical, biotechnology, agrochemical, cosmetics, and food industry companies and to academic and regulatory agencies worldwide for use in the conduct of industry-based research. The Company is headquartered in Southern California, with offices in Buffalo, NY, Research Triangle Park, NC, and Paris, France. Our common stock has traded on the Nasdaq Global Select Market under the symbol “SLP” since May 13, 2021, prior to which it traded on the Nasdaq Capital Market under the same symbol.
We are a global leader, delivering relevant, cost-effective software and creative and insightful consulting services. Pharmaceutical and biotechnology companies use our software programs and scientific consulting services to guide early drug discovery (molecule design screening and lead optimization), preclinical, and clinical development programs, and development of generic medicines after patent expiration, including using our software products and services to enhance their understanding of the properties of potential new medicines and to use emerging data to improve formulations, select and justify dosing regimens, support the generics industry, optimize clinical trial designs, and simulate outcomes in special populations, such as in elderly and pediatric patients.
SEGMENT INFORMATION
During the year ended August 31, 2021, our business was organized into four reportable segments, based on the following business divisions: (i) Simulations Plus, (ii) Cognigen, (iii) DILIsym, and (iv) Lixoft.
Simulations Plus
Simulations Plus is focused on improving the way scientists use knowledge and data to predict the properties and outcomes of pharmaceutical and biotechnology agents by providing a wide range of early discovery, preclinical, and clinical consulting services and software. Our innovations in integrating new and existing science in medicinal and computational chemistry, pharmaceutical science, biology, physiology, AI and machine learning into our software have enabled us to be a leading software provider for modeling and simulation, and prediction of molecular properties from structure.
Cognigen
We acquired Cognigen Corporation (“Cognigen”) in September 2014. With the acquisition of Cognigen, the Company became a leading provider of population modeling and simulation contract research services for the pharmaceutical and biotechnology industries. Our consulting services include modeling, clinical trial simulations, data programming, and technical writing services in support of regulatory submissions.
Effective September 1, 2021, we merged Cognigen with and into Simulations Plus, Inc. through a short-form merger (the “Cognigen Merger”). To effectuate the Cognigen Merger, we filed Certificates of Ownership with the Secretaries of State of the states of Delaware (Cognigen’s state of incorporation) and California (the Company’s state of incorporation). Consummation of the Cognigen Merger was not subject to approval of our stockholders and did not materially impact the rights of our stockholders.
| 2 |
DILIsym
We acquired DILIsym Services, Inc. (“DILIsym”) in June 2017. The acquisition of DILIsym positioned the Company as a leading provider of Quantitative Systems Toxicology services including Drug Induced Liver Injury (“DILI”) modeling and related consulting services as well as simulation programs for analyzing nonalcoholic fatty liver disease (NAFLD). DILIsym has subsequently expanded its capabilities to include Quantitative Systems Pharmacology services in areas including idiopathic pulmonary fibrosis (IPF) and Radasym.
Effective September 1, 2021, we merged DILIsym with and into Simulations Plus, Inc. through a short-form merger (the “DILIsym Merger”). To effectuate the DILIsym Merger, we filed Certificates of Ownership with the Secretaries of State of the states of Delaware (DILIsym’s state of incorporation) and California (the Company’s state of incorporation). Consummation of the DILIsym Merger was not subject to approval of our stockholders, and did not materially impact the rights of our stockholders.
Lixoft
We acquired Lixoft as a wholly owned subsidiary in April 2020. Lixoft brings to the Company MonolixSuite®, which can take modeling projects from data exploration to clinical trial simulations. In addition, Lixoft provides training and consulting services designed to accelerate pharmacometric studies. Lixoft’s technologies were developed as a result of a research program led by the French national research institute for digital science and technology (Inria).
Below is a summary of revenue percentages by reportable segment for the fiscal years ended August 31:
| 2021 | 2020 | 2019 | ||||||||||
| Simulations Plus, Inc. | 54% | 53% | 58% | |||||||||
| Cognigen | 23% | 26% | 27% | |||||||||
| DILIsym | 13% | 17% | 15% | |||||||||
| Lixoft | 10% | 4% | —% | |||||||||
| Total | 100% | 100% | 100% | |||||||||
Going forward, we expect to report segment information based on our two business units (software business and consulting services business) in addition to reporting by division.
PRODUCTS
General
We currently offer twelve software products for pharmaceutical research and development as follows:
| · | Three simulation products that provide time-dependent results based on solving large sets of differential equations: |
| o | GastroPlus® |
| o | DDDPlus™ |
| o | MembranePlus™ |
| · | Two products that predict and analyze static properties of chemicals utilizing both artificial intelligence as well as machine learning technologies: |
| o | ADMET Predictor® |
| o | MedChem Designer™ |
| 3 |
| · | Five products that are based on mechanistic, mathematical models: |
| o | DILIsym® |
| o | NAFLDsym® |
| o | IPFsym™ |
| o | RENAsym® |
| o | MITOsym® |
| · | Two products designed for modeling and simulation that allow for population analyses and rapid clinical trial data analysis and regulatory submissions: |
| o | MonolixSuite® (the combination of Monolix, PKanalix, and Simulx). |
| o | PKPlus™ |
Software Business
Our software business represented 60% of our total revenue during the year ended August 31, 2021, and was primarily generated by the following products:
GastroPlus
Our flagship product, originally introduced in 1998, and currently our largest single source of software revenue, is GastroPlus. GastroPlus mechanistically simulates the absorption and drug interaction of compounds administered to humans and animals and is currently one of the most widely used commercial software products of its type by industry and regulatory agencies in the U.S. and globally. Our goal with GastroPlus is to integrate the most advanced science into user-friendly software to enable researchers and regulators to perform sophisticated analyses of complex compound behaviors in humans and laboratory animals. We work to release updated versions of the program on an ongoing basis.
In February 2021, GastroPlus version 9.8.1, which included new mechanisms and updated documentation for key drug interaction standards models, was released. This version added important new capabilities, including improvements to population simulations, additional dosage route models, drug interactions, and new enhancements when importing chemical structures, among others.
Because of the widespread use of GastroPlus, we have been able to enter into both funded and unfunded collaborations with industry and government agencies to drive advances to modeling and simulation science. In all such collaborations, we own the intellectual property developed within the GastroPlus program, and updates are integrated into future versions and made available to clients. Recent collaborations include:
| · | Virtual bioequivalence trials: in October 2019, we entered a funded collaboration with a large pharmaceutical company to develop the Virtual Bioequivalence (“BE”) Trial Simulator™ in GastroPlus. This collaboration enhances the GastroPlus platform to evaluate population and formulation variability on the BE of different products. |
| · | Oral absorption – Advanced Compartmental Absorption and Transit (ACAT™) model: in November 2019, we entered a new funded collaboration with a large pharmaceutical company to modify the mechanistic ACAT™ model in GastroPlus to support gastrointestinal disease research. In January 2021, we entered into a new funded collaboration with a different large pharmaceutical company to add novel mechanisms for oral peptide formulations within the ACAT™ model to expand oral absorption modeling beyond small molecules. |
| · | Oral cavity absorption model: in October 2020, through a joint proposal with the St. Louis College of Pharmacy at University of Health Sciences and Pharmacy in St. Louis, we were awarded a new funded cooperative agreement from the FDA to establish novel in vitro/in silico models for the oral cavity route of administration in GastroPlus. |
| · | Unfunded research collaborations: We also have two unfunded RCAs with the FDA: one with the Office of Generic Drugs (“OGD”) that began in 2014, and one announced in July 2019 with the Center for Veterinary Medicine (“CVM”). |
| 4 |
ADMET Predictor
ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) Predictor is a top-ranked, chemistry-based computer program that takes molecular structures as inputs and uses machine learning technology to predict different properties for them. This capability allows chemists to generate estimates for many important molecular properties without the need to synthesize and test the molecules. A chemist can then assess the likely success of many existing molecules in a company’s chemical library, as well as molecules that have never been made.
The optional ADMET Modeler™ AIDD (Artificial Intelligence-driven Drug Design) Module in ADMET Predictor enables scientists to use their own experimental data to quickly create proprietary high-quality predictive models using the same powerful AI engine we use to build our top-ranked property predictions.
Version 10.2 of ADMET Predictor marketed as APX.2, was released in April 2021, which added many new features including, but not limited to: (i) new capabilities in the High Throughput Pharmacokinetic (“HTPK”) Simulation Module which integrates machine learning and physiologically based pharmacokinetic (PBPK) technologies to guide lead selection, (ii) new intravenous (IV) bolus route of administration to complement oral dosing options, (iii) Multi-dosing for more complex administration regimens, (iv) plasma concentration versus time curve overlay for easy comparison across compounds, (v) Additional options for dose optimization to support universal safety and efficacy definitions.
We have made significant investments in two key areas with recent versions: improving integration of our top-ranked ADMET Predictor and GastroPlus models to leverage our novel ‘Discovery PBPK’ approaches for chemists and safety researchers, and further enhancing our best-in-class machine learning engine to assist with drug discovery.
Recent collaborations include:
| · | Drug discovery workflows: in December 2019, we entered into a new collaboration agreement with Bayer AG to advance our ADMET Predictor machine learning software for use within integrated drug discovery workflows by developing improved structure and tautomer handling capabilities that will support data integrity across the different discovery platforms. |
| · | High-throughput pharmacokinetic (HTPK) simulations: in April 2020, we entered into a new collaboration agreement with a large pharmaceutical company to develop enhanced capabilities in our existing HTPK Simulation Module which will incorporate PBPK modeling into the partner’s discovery platform to support compound screening activities. In September 2020, we entered an accelerated second phase of this collaboration with the sponsor company to add further enhancements to the HTPK Simulation Module calculations and workflows. |
| · | Artificial Intelligence Drug Discovery (“AIDD”) Module validation: in September 2020, we entered into a collaborative research agreement with a large pharmaceutical company to apply the AIDD Module to an active therapeutic program. We worked with the partner to define the multi-objective parameters against which the lead molecule(s) were to be optimized. In April 2021, we received initial experimental results from 10 candidate molecules selected for synthesis and testing. In August 2021, the second phase of experimental results were received. Peer-reviewed manuscripts of the methodology and results are expected to be jointly published with our collaboration partner in 2022. |
MonolixSuite
The MonolixSuite is a unique solution for modeling and simulation for pharmaceutical companies, biotechnology enterprises, and hospitals. It supports nonparametric analyses, population analyses and modeling, and clinical trial simulation. The extended MonolixSuite contains three main products: Monolix, Simulx, and PKanalix. Monolix 2020R1 was released in November 2020, which combines the most advanced algorithms with a unique ease of use. The products are used by pharmaceutical companies across the globe at each step of drug development, from preclinical to first-in-human, clinical, and post-approval.
| 5 |
Consulting Services Business
General
Our scientists and engineers have extensive expertise in drug absorption via various dosing routes, pharmacokinetics, pharmacodynamics, drug-drug interactions and other areas related to the drug development process. We conduct contracted consulting studies for large customers with complex problems and who recognize our expertise in solving them, as well as for smaller customers. The demand for our consulting services has been steadily increasing, and we have expanded our consulting teams to meet the increased workload.
Our consulting services business represented 40% of our total revenue during the year ended August 31, 2021, and was primarily generated by the following services:
PBPK
Beginning in 2014, the FDA and other regulatory agencies began to emphasize the need to encourage mechanistic PBPK modeling and simulation in clinical pharmacology, with final guidance documents completed in 2018. New draft guidance documents, which were released in October 2020 focused on additional biopharmaceutics applications for oral drug product development, manufacturing changes, and controls. This has resulted in an increased need for our scientific consulting staff to drawn upon its extensive experience across multiple therapeutic areas of modeling and simulation methods to provide consulting-related services in support of this sophisticated technique. We support Model-Informed Drug Discovery and Development throughout the entire product lifecycle, from discovery through translational research and clinical development when an organization does not have the time or resources to use our software directly. More specifically, our clients seek out our consulting services to acquire scientific, therapeutic-area-related modeling and simulation expertise that they do not have in-house.
PKPD
Our clinical-pharmacology-based consulting services include population pharmacokinetic and pharmacodynamic modeling, exposure-response analyses, clinical trial simulations, data programming, and technical writing services in support of regulatory submissions. In addition to modeling and simulation consulting services, we provide expertise and assistance with development-related decision-making and support for regulatory interactions related to dose selection, clinical trial design, and understanding of the determinants of safety and efficacy for new medicines.
QSP/T
We provide creative and insightful consulting services to support our quantitative systems pharmacology/quantitative systems toxicology (“QSP/QST”) modeling focused on nonalcoholic fatty liver safety NAFLD, and nonalcoholic steatohepatitis (“NASH”), IPF, heart disease, liver and kidney safety, and radiation syndrome, as well as other areas.
| 6 |
SALES AND MARKETING
We market our software and consulting services globally through attendance and presentations at scientific meetings, exhibits at trade shows, seminars at pharmaceutical companies and government agencies, our website, and various communication channels to our database of prospects and customers. At various scientific meetings around the world each year there are numerous presentations and posters reporting research that was performed using our software. Many of these presentations are from industry and FDA scientists; some are from our staff. Numerous peer-reviewed scientific journal articles are published, and conference presentations delivered, each year using our software, mostly by our customers, further supporting its use in a wide range of preclinical and clinical studies.
Our sales and marketing efforts are handled primarily internally by sales and marketing staff and with our scientific team and several senior management staff assisting our marketing and sales staff with trade shows, seminars, and customer training both online and on-site. We also have independent distributors in Japan, China, India, and Korea as well as in South America who sell and market our products with support from our scientists and engineers.
We also launched our MIDD+ (Model-Informed Drug Development) scientific conference in March 2021, where speakers shared their real-world impact using modeling & simulation technology. During the two-day event, representatives from the U.S. FDA Offices of Clinical Pharmacology, New Drug Products, Research and Standards, Translational Sciences, the Center for Drug Evaluation and Research, and the National Center for Toxicological Research, as well as ANVISA and Health Canada, provided case studies and software demonstrations on wide range of topics. The event also featured a panel discussion on the ascent of model-informed drug development and the increasing importance of developing next generation technology.
COMPETITION
We compete against a number of established companies that provide screening, testing, and research services, and products that are not based on simulation software. There are also software companies whose products do not compete directly with us but are sometimes closely related to, ours. Our competitors in this field include some companies with financial, personnel, research, and marketing resources that are larger than ours.
Major pharmaceutical companies conduct drug discovery and development efforts through their internal development staff and outsourcing. Smaller companies generally need to outsource a greater percentage of this effort. Thus, we compete not only with other software suppliers and scientific consulting service providers but also with the in-house development and scientific consulting teams at some of the larger pharmaceutical companies.
Based on our technical knowledge and expertise, we believe that we are strategically positioned to offer competitive modeling and simulation consulting services to companies. Our clients seek out our services for multiple reasons including, without limitation: (i) to acquire scientific, therapeutic-area-related modeling expertise that they do not have in-house, (ii) to address a need for modeling and simulation efforts beyond the capacity of in-house resources, (iii) to fulfill their modeling requirements more efficiently than they could do in-house, and (iv) to utilize our software when they do not have the in-house expertise to do so. We apply our software and assist companies in such areas as physiologically based pharmacokinetic modeling (“PBPK”), pharmacokinetic/pharmacodynamic (PK/PD) data analysis, and quantitative systems pharmacology/toxicology (QSP/QST). We compete against numerous service providers, ranging from departments within large contract research organizations (“CROs”) to independent consulting organizations of various sizes as well as individual consultants.
We believe the key factors in our ability to successfully compete in this field are our ability to: (i) continue to invest in research and development, and develop and support industry-leading simulation and modeling software and related products and services, (ii) develop and maintain a proprietary database of results of physical experiments that serve as a basis for simulated studies and empirical models, (iii) continue to attract and retain a highly-skilled scientific and engineering team, (iv) aggressively promote our products and services to our global market, and (v) develop and maintain relationships with research and development departments of pharmaceutical companies, universities, and government agencies.
In addition, we actively seek strategic acquisitions to expand both our pharmaceutical software portfolio and services offerings.
| 7 |
TRAINING AND TECHNICAL SUPPORT
Customer training and technical support are important factors in customer satisfaction for our pharmaceutical products, and we believe we are an industry leader in providing strong customer training and technical support in our business areas. We provide in-house seminars at customers’ and potential customers’ sites, as well as at selected universities to train students who will soon be industry scientists. These seminars often serve as initial training in the event the potential customer decides to license or evaluate our software. Technical support is provided after the sale of any software in the form of on-site training (at the customer’s expense), web meetings and telephone, fax, and e-mail assistance to the customer’s users during the customer’s license period.
We provide telephone, e-mail, and web-based support for all of our software products. Technical support for our software is provided by our life sciences teams and our inside sales and support staff. Technical support for our software products is generally minimal.
We provide support to the GastroPlus User Group in Japan, which was organized by Japanese researchers in 2009. In early 2013, a group of scientists in Europe and North America organized another GastroPlus User Group following the example set in Japan. Over 1,300 members have joined this group to date. We support this group through coordination of online meetings each month and managing the user group website for exchange of information among members. These user groups provide us valuable feedback for desired new features and suggested interface changes.
RESEARCH AND DEVELOPMENT
The development of our software is focused on expanding our product portfolio, designing enhancements to our core technologies, and integrating existing and new products into our principal software architecture and platform technologies. We intend to continue to offer regular updates to our products and to continue to look for opportunities to expand our existing suite of products and services.
To date, we have developed products internally, sometimes also licensing or acquiring products, or portions of products, from third parties. In certain instances, these arrangements have required that we pay royalties to third parties; we have paid no royalties during the year ended August 31, 2021. We intend to continue to license or otherwise acquire technology or products from third parties when it makes business sense to do so.
Research and development (“R&D”) activities include both enhancement of existing products and development of new products. Development of new products and adding functionality to existing products are capitalized in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 985-20, “Costs of Software to Be Sold, Leased, or Marketed.” R&D expenditures, which primarily relate to both capitalized and expensed salaries, R&D supplies, laboratory testing, and R&D consulting, were approximately $6.9 million during fiscal year 2021, of which $2.9 million was capitalized. R&D expenditures were approximately $5.3 million during fiscal year 2020, of which $2.3 million was capitalized. R&D expenditures during fiscal year 2019 were approximately $4.3 million of which $1.8 million was capitalized.
Our software products are designed and developed by our development teams managed out of offices in California, North Carolina (Research Triangle Park), New York (Buffalo), and Paris, France. We also employ people who can work remotely using collaboration software. Our products and services are delivered electronically.
CUSTOMERS
Our customers include biotech and pharmaceutical companies, universities, and regulatory agencies and other government organizations. We concentrate on serving the needs of our customers in drug discovery, development, clinical trials, and post-patent generic formulation development. Our current customer base is highly fragmented; in 2021, three of our customers each accounted for 11%, 4% and 3% of our revenues, respectively. With the one exception, no other customers made up more than 11% of our revenues in the last four years.
| 8 |
SEASONALITY
Our revenues exhibit seasonal fluctuations, with the first fiscal quarter (September-November) and fourth fiscal quarter (June-August) generally having the lowest revenues due to vacations and reduced activities at our customers’ sites. In addition, as revenue has grown in recent years, we have seen some flattening from quarter to quarter due to higher revenue in our second and third quarters. This is due to pharmaceutical industry buying patterns as well as our revenue recognition policies for software, consulting service slowdowns due to vacations, and lower customer and employee conference attendance in those periods. Revenues for any quarter are not necessarily indicative of revenues for any future period; however, because our pharmaceutical software is licensed on an annual basis, renewals are usually within the same quarter year after year.
ENVIRONMENTAL MATTERS
We believe we are in compliance in all material respects with all applicable environmental laws. Presently, we do not anticipate that such compliance will have a material effect on capital expenditures, earnings, or competitive position with respect to any of our operations.
HUMAN CAPITAL RESOURCES
We are committed to our people, and we embrace a culture of engagement, empowerment, and equity. Over 90 percent of our global employees are employed full-time, and more than two-thirds work within our life sciences software or consulting divisions. Given the specialized nature of our business, candidates for our open positions are strategically selected for their unique education and skills. The majority of our employees have advanced degrees in mathematics, chemistry, biomedical engineering, and/or the pharmaceutical sciences; more than half hold doctorate degrees and approximately one-fifth hold master’s degrees.
As of August 31, 2021, we employed a total of 146 persons, including 135 full-time employees and 11 part-time employees, consisting of 92 in scientific, technical, and research and development, 10 in marketing and sales, and 44 in administration and accounting. Currently 73 employees hold PhDs. (including PharmDs) in their respective science or engineering disciplines, and 31 employees hold one or more Master’s degrees. Most of the senior management team and all of the members of our Board of Directors hold graduate degrees.
We believe that our future success will depend, in part, on our ability to continue to attract, hire, and retain qualified personnel. We continue to seek additions to our science and technical staff, although the competition for such personnel in the pharmaceutical industry is intense. None of our employees is represented by a labor union, and we have never experienced a work stoppage. We believe that our relations with our employees are good.
Diversity, Equity and Inclusion
In 2020, we expanded our Human Resources team to implement unified and consistent policies, procedures, and employee training across all of our business units. In our recruitment and hiring, we embrace diversity with the knowledge that it can lead to greater innovation, and in our workplace, we foster inclusion so all employees feel they are a part of our team with equal access to all opportunities. One of our goals is to continue expanding our focus on diversity, equity and inclusion. In terms of gender equity, women comprise 46% of our workforce and men comprise 54%.
Training and Awareness Programs
In 2020, we expanded our Human Resources team and broadened our capabilities to include, among other responsibilities, a new focus on training and development. Company-wide, we have analyzed all of our positions, including job descriptions and salary bands, and are creating career paths for the different functions within our organization. We will use these career paths as a basis for promoting employee career development and growth within the organization, as well as in recruiting and hiring new talent.
As we finalize the career paths, we are also committed to conducting a Talent Gap Analysis, a process by which we will design and implement a comprehensive training program to be integrated into the annual Performance Review cycle. This employee training program will include several key components of career development such as technical and soft skills, leadership development, and mandated employee compliance training.
| 9 |
In addition to these new employee training and development initiatives, we have an ongoing program of cross-specialty training consisting of presentations by expert modelers from each division. These monthly sessions serve to familiarize all divisions with the applications and techniques unique to each division and, in so doing, create opportunities to find synergies, expand the knowledge base across all divisions, and build a shared sense of purpose.
Health & Safety
We place a high value on maintaining a clean, safe, and healthy environment for our employees. We believe that we have in place effective procedures to identify, evaluate, and mitigate potential risks associated with our operations, although we believe such risks are minimal.
The well-being of our employees, whether they are working in our divisional offices or remotely from home offices, is paramount. We believe that we are substantially in compliance with all applicable laws, regulations, and standards, and we make every effort to be attentive and responsive to our employees’ needs. In our offices, we have provided employees with ergonomic equipment, including ergonomic chairs and standing desks, and for their home offices, we provide an allowance for the purchase of home office equipment.
We also consider open and transparent channels of communication to be as a critical component of our employee health and wellness program. Toward this end, on a quarterly basis, we hold a company-wide virtual meeting to keep our employees engaged, informed, and apprised of activities occurring at the company and at each division, including quarterly financial results, future goals, and notable milestones.
INTELLECTUAL PROPERTY AND OTHER PROPRIETARY RIGHTS
We primarily protect our intellectual property through copyrights and trade secrets. Our intellectual property consists primarily of source code for computer programs and data files for various applications of those programs in the pharmaceutical software businesses. The expertise of our staff is a considerable asset closely related to intellectual property, and attracting and retaining highly qualified scientists and engineers is essential to our business.
EFFECT OF GOVERNMENT REGULATIONS
We believe that our operations are substantially in compliance with all applicable laws and regulations and that it holds all necessary permits to operate our business in each jurisdiction in which our facilities are located. Laws and government regulations are subject to change and interpretation. Our pharmaceutical software products are tools used in research and development and are neither approved nor approvable by the FDA or other government agencies.
No significant pollution or other types of hazardous emission result from our operations and it is not anticipated that our operations will be materially affected by federal, state or local provisions concerning environmental controls. Our costs of complying with environmental, health and safety requirements have not been material. Furthermore, compliance with federal, state and local requirements regulating the discharge of materials into the environment, or otherwise relating to the protection of the environment, have not had, nor are they expected to have, any material effect on the capital expenditures, earnings or competitive position of the Company.
COMPANY WEBSITE
We maintain a corporate website at: www.simulations-plus.com.
The contents of this website are not incorporated in or otherwise to be regarded as part of this Annual Report. We file reports with the SEC which are available on our website free of charge. These reports include annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, “Section 16” filings on Form 3, Form 4, and Form 5, and other related filings, each of which is provided on our website as soon as reasonably practical after we electronically file such materials with or furnish them to the SEC. In addition, the SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including the Company.
| 10 |
ENVIRONMENTAL, SOCIAL, GOVERNANCE
We are committed to providing consistent and excellent return to our shareholders, all while maintaining a strong sense of good corporate citizenship that places a high value on the welfare of our employees, the communities in which we operate, and the world as a whole. We believe that effectively prioritizing and managing our Environmental, Social, and Governance (“ESG”) topics will help create long-term value for our investors. We also believe that transparently disclosing the goals and relevant metrics related to our ESG programs will allow our stakeholders to be informed about our progress.
The topics covered in this section are identified through third-party ESG reporting frameworks, standards and metrics, such as the Sustainability Accounting Standards Board (“SASB”), and United Nations Sustainable Development Goals. More information on our key ESG programs, goals and commitments, and key metrics can be found on our website in our 2020 ESG Report.
Our ESG highlights include the following:
COVID-19 Response
With employee health and safety always a top priority, we proactively implemented a COVID-19 Contingency Plan in late February of 2020, prior to the state-issued stay-at-home orders. The comprehensive plan included information on prevention measures, travel restrictions, when and how to quarantine, the Families First Coronavirus Response Act, sick leave arrangements, including caring for family members affected by COVID-19, and workplace safety measures. At the time, as part of our ongoing flexible work initiative to give employees the option of telecommuting or working remotely, over 40 percent of our workforce was already working from home, however in response to the COVID-19 pandemic, we took quick action to ensure the safety of the rest of our workforce by supporting them in setting up home offices.
Since that initial plan was disseminated, additional updates from management have included the most up-to-date information from the U.S. Department of State, Center for Disease Control (‘CDC”) and World Health Organization (“WHO”), and we have, at all times, encouraged employees to keep management informed of the need for any additional support. Our COVID-19 Contingency Plan communication and our Policy for Returning to Work During the Coronavirus Pandemic specify several CDC-recommended measures to mitigate the spread of COVID-19 in the workplace, including that masks be worn in the office, the importance of social and physical distancing and frequent hand-washing, and that employees are to remain home if feeling unwell and self-quarantine following any possible exposure to the virus. In addition to these measures, we have increased sanitation procedures and updated our travel policy to ensure the safety of those employees who have resumed working in the office and those who travel for business.
We will continue to monitor mandates, guidelines and recommendations issued by the CDC, WHO and local governments as they are released, and revise our COVID-19 Contingency Plan communication and our Policy for Returning to Work During the Coronavirus Pandemic accordingly.
Environmental Matters
| · | We participate in a recycling program through our local waste management facility to divert all recyclable materials – bottles, cans, plastics, paper, and cardboard – from landfills. Across the Company, our facilities provide for recycling, and our electronic waste is sent to local approved e-waste recycling centers. |
| · | Our operations are built on continual improvements in efficiency and clean energy. From 2012 to 2019, our Cognigen division redesigned its data center to be more energy efficient as part of our ongoing and increasing commitment to reduce our environmental footprint and energy usage. An example of an upgrade is the installation of an uninterruptible power supply with hot and cold dial separation, and regulating the temperature and airflow through in-row cooling units with high efficiency fans based on cooling needs. |
| · | We are also attentive to our energy use in our office operations. For instance, our Lancaster facility recently switched to renewable energy. Lancaster Choice Energy (“LCE”) is the locally run power program created by the City of Lancaster, and we now proudly participate in LCE’s Smart Choice 100% renewable energy program. Our decision to opt in to the program not only contributes to the city’s goal of becoming one of the world’s first net-zero cities, but also reflects our dedication to creating positive impacts on the environment and local communities. |
| · | We believe we are in compliance in all material respects with all applicable environmental laws. Presently, we do not anticipate that such compliance will have a material effect on capital expenditures, earnings, or competitive position with respect to any of our operations. |
| 11 |
Social Impact and Supporting our Communities
| · | Our support for the academic community is broad and deep. We provide certain distinguished professors at academic institutions with free reference site licenses for nonprofit research and teaching, including providing free access to our software in university instruction. In addition to reference site licenses, academic and research institutions are entitled to a 95% discount off commercial license fees, and we offer students and professors either free or substantially reduced fees to attend our training courses and workshops. In recent years, we have sponsored several students with awards given by the Society of Toxicology. |
| · | We provide sponsorships to numerous conferences, symposia, and associations such as the American Conference on Pharmacometrics (ACoP), American Association of Pharmaceutical Scientists (AAPS), American Chemical Society (ACS), Controlled Release Society (CRS), Groupe de Métabolisme et Pharmacocinétique (GMP), and the Gordon Research Conferences. |
| · | At the local level, we promote a culture of voluntarism, and we offer our employees the flexibility they need to participate, from sponsoring and participating in charity golf tournaments to volunteering to serve hot meals to the disadvantaged. In recent years, we have joined the global GivingTuesday movement and donated food, clothing, and financial support to several organizations that serve those in need in our communities. |
Our People
| · | Our commitment to community, to education, and to gender equity can best be summarized by how we have, for more than a decade, funded a summer scholarship to Tech Trek, a one-week residential science, technology, engineering, and math (“STEM”) camp founded and operated by the American Association of University Women (AAUW) that is designed to inspire young women to attend college, to major in STEM fields, and to pursue STEM careers. Our own female scientists, who are excellent role models for these young women, have volunteered their time to personally present our Tech Trek scholarship each year. |
Customer Privacy & Data Security
| · | We value customer privacy and the data we collect are only as needed to deliver company information, software products, and consulting services. Our website includes our comprehensive Privacy Policy, which details what and how data are collected, how data are used and stored, and the options for controlling personal data, including opting-out, accessing, updating, or deleting it. |
| · | In recognition of the critical importance of Data Security to our operations, including Cybersecurity, Data Protection and Customer Privacy, our executive leadership team conducts a thorough examination of all elements of Data Security. Our objective is to ensure the security, confidentiality, and privacy of our systems and information assets, and to follow and be compliant with all relevant laws, regulations, and guidelines, including, but not limited to: |
| o | U.S. and State Data Privacy Laws | |
| o | The EU’s General Data Protection Regulation (“GDPR”) | |
| o | Pharmaceutical Good Practice Quality Guidelines, including FDA 21 CFR Part 11 | |
| o | Sarbanes-Oxley Act | |
| o | Personal Information Protection Law of the People’s Republic of China (“PIPL”) |
| · | Our corporate-level IT department brings greater consistency, efficiency, and functional IT support across all divisions. The IT department is responsible for centralizing divisional data processing, storage, and backup capabilities at each of our geographical locations. The IT department is also responsible for ensuring that corporate IT policies are aligned and compliant with all applicable regulatory provisions and current best practices. |
| 12 |
| · | Our corporate-level Data Protection Officer (DPO) is responsible for establishing and maintaining a Personal Data Privacy program throughout the Company that is compliant with applicable data privacy laws and legislation at the state and federal levels, as well as the EU’s GDPR, and China’s PIPL. The DPO is leading our efforts to further build and implement a company-wide Personal Data Protection and Customer Privacy framework, protocols, and training. |
| · | Our ongoing program of employee training in security awareness keeps our staff fully informed about potential cyber threats - such as phishing and malware – with periodic random phishing tests. |
Business Ethics
| · | From the Company’s inception, we have placed the highest emphasis on conducting our business with honesty and integrity. The highest ethical standards are expected of management and employees alike, and we continuously strive to create a corporate culture of honesty, integrity, and trust. Throughout our operations and in our dealings with our stakeholders, we endeavor to engender the confidence that the Company’s conduct is beyond reproach. |
| · | The policies we have developed are intended to: |
| o | Define and disseminate our core values and the legal requirements applicable to good business conduct and ethical behavior | |
| o | Offer guidance in understanding Company policies, interpreting laws, and handling Company-related issues and situations | |
| o | Foster clear, ethical behaviors and conduct to create an atmosphere of respect, trust, cooperation, and collaboration throughout the Company and its activities | |
| o | Provide clear and well-defined procedures by which employees can easily obtain information, ask questions, and, if necessary, report any suspected violations of any of our Business Ethics policies |
| · | In addition to abiding by all applicable laws, all management and employees are required to comply fully with our Corporate Code of Business Conduct and Ethics (“CCBCE”) which sets forth the Company’s values, business culture, and practices. |
Human Rights
| · | The Company was founded on the belief that our software technologies could lead to important advances in healthcare, thereby improving patient outcomes, advancing and improving global health, and bettering the lives of humankind. This objective cannot be accomplished without a commitment to Human Rights, and we are committed to ensuring that, in our day-to-day business practices, in our business relationships, and in matters of employment, we will uphold our own principles as delineated in our Corporate Code of Business Conduct and Ethics. Furthermore, we support the principles set forth in the United Nations International Bill of Human Rights, specifically the Universal Declaration of Human Rights, and the ILO Declaration on Fundamental Principles and Rights at Work. As we evolve this policy, we will look to the UN Guiding Principles on Business and Human Rights (UNGPs) for guidance. |
Governance
| · | We are committed to ensuring strong corporate governance practices on behalf of our shareholders and other stakeholders. We believe strong corporate governance provides the foundation for financial integrity and shareholder confidence. Our Board of Directors is responsible for the oversight of risks facing the Company, while our management is responsible for the day-to-day management of risk. The Board, as a whole, directly oversees our strategic and business risk, including risks related to financial reporting, compensation practices, ESG, and product developments. More information about our corporate governance features can be found in our Proxy Statement for the 2022 Annual Meeting of Shareholders (the “Proxy Statement”), which will be filed within 120 days after August 31, 2021, the close of our fiscal year covered by this Annual Report. |
| 13 |
ITEM 1A – RISK FACTORS
You should carefully consider the risks described below, as well as the other information in this Annual Report on Form 10-K, including our financial statements and the related notes and the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before investing in our publicly traded securities. The occurrence of any of the events or developments described below could harm our business, financial condition, operating results, and/or growth prospects. The risks described below are not the only ones facing us. Our business is also subject to the risks that affect many other companies, such as competition, technological obsolescence, labor relations, general economic conditions, geopolitical changes, and international operations. We operate in a rapidly changing environment that involves a number of risks, some of which are beyond our control. Additional risks not currently known to us or that we currently believe are immaterial also may impair our business operations and our liquidity. The risks described below could cause our actual results to differ materially from those contained in the forward-looking statements we have made in this Annual Report on Form 10-K, the information incorporated herein by reference, and those forward-looking statements we may make from time to time. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties.
Risk Factor Summary
Below is a summary of the principal factors that make an investment in our common stock speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below and should be carefully considered, together with other information included in this prospectus.
| · | Our business is subject to risks arising from epidemic diseases, such as the recent outbreak of the COVID-19 illness. | |
| · | Our ability to sustain or increase revenues will depend upon our success in entering new markets, continuing to increase our customer base, and in deriving additional revenues from our existing customers. | |
| · | Consolidation and increasing competition within the pharmaceutical and biotechnology industries, drug development and services industry, and the life science market for modeling and simulation software and for cheminformatics products may decrease the number of our customers and/or affect demand for our products and services. | |
| · |
Increasing competition and increasing costs within the pharmaceutical and biotechnology industries may affect the demand for our products and services, which may affect our results of operations and financial condition. | |
| · | Health care reform and restrictions on reimbursement may affect the companies that purchase or license our products or services, which may affect our results of operations and financial condition. | |
| · | We face strong competition in the life science market for modeling and simulation software and for cheminformatics products. | |
| · | We are subject to pricing pressures in some of the markets we serve. | |
| · | Our operations may be interrupted by the occurrence of a natural disaster or other catastrophic event at our primary facilities. | |
| · | Our insurance coverage may not be sufficient to avoid material impact on our financial position or results of operations resulting from claims or liabilities against us, and we may not be able to obtain insurance coverage in the future. | |
| · | Changes in government regulation or in practices relating to the pharmaceutical or biotechnology industries could decrease the need for the services we provide. | |
| · | Any negative commentaries made by any regulatory agencies or any failure by us to comply with applicable regulations and related guidance could harm our reputation and operating results, and compliance with new regulations and guidance may result in additional costs. | |
| · | Our sales cycle is lengthy and customers may delay entering into contracts or decide not to adopt our products or solutions after we have expended significant time and resources and supported evaluation by them of our technology, which could result in delays in recognizing revenue and negatively impact our results of operations. | |
| · | Many of our contracts are fixed-price and may be delayed or terminated or reduced in scope for reasons beyond our control, or we may underprice or overrun cost estimates with these contracts, potentially resulting in financial losses. | |
| · |
We could experience a breach of the confidentiality of the information we hold or of the security of our computer systems. | |
| · | Impairment of goodwill or intangible assets may adversely impact future results of operations. |
| 14 |
| · | Software defects or malfunctions in our products could hurt our reputation among our customers, result in delayed or lost revenue, and expose us to liability. | |
| · | Delays in the release of new or enhanced products or services or undetected errors in our products or services may result in increased cost to us, delayed market acceptance of our products, and delayed or lost revenue. | |
| · | We are subject to risks associated with the operation of a global business. | |
| · | The drug discovery and development services industry is highly competitive. | |
| · | Changes in applicable U.S. and international tax laws or regulations and the resolution of tax disputes could negatively affect our financial results. | |
| · | Contract research services create a risk of liability. | |
| · | Upgrading our software could result in implementation issues and business disruptions. | |
| · | The drug discovery and development industry has a history of patent and other intellectual property litigation, and we might be involved in costly intellectual property lawsuits. | |
| · | We may not be able to successfully develop and market new services and products. | |
| · | Ability to incur debt could adversely affect our business and growth prospects. | |
| · | We depend on key personnel and may not be able to retain these employees or recruit additional qualified personnel, which could harm our business. | |
| · | If we are not successful in selecting and integrating the businesses and technologies we acquire, or in managing our current and future divestitures, our business may suffer. | |
| · | Our quarterly and annual operating results fluctuate and may continue to fluctuate in the future, and if we fail to meet the expectations of analysts or investors, our stock price and the value of your investment could decline substantially. | |
| · | We derive a significant percentage of our revenues from a concentrated group of customers and the loss of more than one of our major customers could materially and adversely affect our business, results of operations or financial condition. | |
| · | We conduct business outside the U.S., which exposes us to foreign currency exchange rate risk, amongst other risk, and could have a negative impact on our financial results. | |
| · | A significant portion of our operating expenses is relatively fixed and planned expenditures are based in part on expectations regarding future revenues. | |
| · | If our customers cancel their contracts or terminate or delay their clinical trials, we may lose or delay revenues and our business may be harmed. | |
| · | If our security is breached, our business could be disrupted, our operating results could be harmed, and customers could be deterred from using our products and services. | |
| · | Any failure by us to properly protect customer data we possess or are deemed to possess, in connection with the conduct of clinical trials, could subject us to significant liability. | |
| · | We rely upon a single internal hosting facility and Amazon Web Services to deliver our solutions to our customers and any disruption of or interference with our hosting systems, operations, or use of the Amazon Web Services could harm our business and results of operations. | |
| · | Defects or errors in our software applications could harm our reputation, result in significant cost to us and impair our ability to market our solutions. | |
| · | If we are not able to reliably meet our data storage and management requirements, or if we experience any failure or interruption in the delivery of our services over the Internet, customer satisfaction and our reputation could be harmed and customer contracts may be terminated. | |
| · | Some of our software solutions and services utilize open source software, and any failure to comply with the terms of one or more of these open source licenses could adversely affect our business. | |
| · | We may be unable to adequately enforce or defend our ownership and use of our intellectual property and other proprietary rights. | |
| · | Current and future litigation against us, which may arise in the ordinary course of our business, could be costly and time-consuming to defend. | |
| · | We could incur substantial costs resulting from product liability claims relating to our products or services or our customers’ use of our products or services. | |
| · | Our business depends on the clinical trial market, and a downturn in this market could cause our revenues to decrease. | |
| · | As a public company, we are obligated to maintain proper and effective internal control over financial reporting. As our business expands both organically and through acquisitions, we may be unable to effectively adapt our current systems to our changing business needs and may fail to develop and maintain an effective system of disclosure controls and internal control over financial reporting which could impair our ability to produce timely and accurate financial statements or comply with applicable laws and regulations. |
| 15 |
| · | As a public company, we may incur significant administrative workload and expenses in connection with new and changing compliance requirements. | |
| · | We have been paying quarterly dividends on shares of our common stock, and although there has been a consistent track record of paying these dividends, the Board of Directors may suspend the dividend, and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock. | |
| · | If our operating and financial performance in any given period does not meet any guidance that we provide to the public, the market price of our common stock may decline. | |
| · | The price of our common stock may fluctuate significantly, and investors could lose all or part of their investments. | |
| · | The price of our common stock may be volatile, and our stockholders may not be able to resell shares of our common stock at or above the price they paid. | |
| · | If securities or industry analysts issue an adverse or misleading opinion regarding our stock, or our inclusion in the S&P 600 discontinues, our stock price and trading volume could decline. | |
| · | We may raise capital through the issuance of our common stock, convertible debt or equity linked securities, which could result in dilution to our stockholders or negatively impact the price of our common stock. |
Certain Risks Related to Our Business
Our business is subject to risks arising from epidemic diseases, such as the recent outbreak of the COVID-19 illness.
We are subject to risks related to public health crises such as the global pandemic associated with COVID-19. In December 2019, a novel strain of coronavirus, SARS-CoV-2, was reported to have surfaced in Wuhan, China. Since then, COVID-19 has spread worldwide and has resulted in government authorities implementing numerous measures to try to contain it, such as travel bans and restrictions, quarantines, shelter-in-place orders and shutdowns. The ongoing COVID-19 global pandemic and variants thereof is having widespread, rapidly-evolving, and unpredictable impacts on global societies, economies, financial markets, and business practices. COVID-19 poses the risk that we or our employees, contractors, suppliers, and other partners may be prevented from conducting business activities for an indefinite period of time, including due to shutdowns that may be requested or mandated by governmental authorities. If the current economic conditions worsen or last for an extended period of time, we may be forced to significantly scale back our business and growth plans, which could have a material adverse effect on our business.
We have undertaken several measures in an effort to mitigate the spread of COVID-19, including adjusting our business practices to combat the effects by restricting employee travel, closing our offices in compliance with local guidelines and, when reopened, implementing social distancing at our office locations and additional sanitary measures. There have been no reductions in our workforce as a result of COVID-19. During the 2021 fiscal year, software renewal revenue and services revenues generated primarily from contracts signed prior to the effects of the pandemic have not been materially impacted. Revenue from new software licenses and new service contracts has been negatively impacted with our clients’ increased focus on opportunities to address COVID-19 mitigation efforts and disruption in drug programs in other therapeutic areas.
While the COVID-19 pandemic has not materially adversely affected our business operations as of the date of this Annual Report on Form 10-K, the continued spread of COVID-19 and variants thereof and the measures taken by the governments of countries affected could disrupt the supply chain and adversely impact our business, financial condition, or results of operations. The COVID-19 outbreak and mitigation measures may also continue to have an adverse impact on global economic conditions, which could have an adverse effect on our business and financial condition. The extent to which the COVID-19 outbreak further impacts our results going forward will depend on future developments that are highly uncertain and cannot be predicted, including but not limited to, the continued duration and spread of the outbreak, the emergence of novel variants, the degree of severity of the outbreak and existing and new variants, the development and administration of existing and new therapeutic treatments and vaccines, the actions taken by national, regional, and local governments and health officials to contain the virus or treat its impact, how quickly and to what extent normal economic and operating conditions can resume, and the extent to which our third-party partners and/or customers experience any business interruptions as a result thereof. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our common stock.
| 16 |
Certain Risks Related to Our Marketplace and Environment
Our ability to sustain or increase revenues will depend upon our success in entering new markets, continuing to increase our customer base, and in deriving additional revenues from our existing customers.
Our products are currently used primarily by modeling and simulation specialists in pharmaceutical, biotechnology, agrotechnology, cosmetics, and government research organizations. One component of our overall business strategy is to derive more revenues from our existing customers by expanding their use of our products and services. Such strategy would have our customers utilize our scientific informatics platforms and our tools and components to leverage vast amounts of information stored in both corporate databases and public data sources in order to make informed scientific and business decisions during the research and development process. In addition, we seek to expand into new markets, and new areas within our existing markets, by acquiring businesses in these markets, attracting and retaining personnel knowledgeable in these markets, identifying the needs of these markets, and developing marketing programs to address these needs. If successfully implemented, these strategies would increase the usage of our software and services by pharmacologists or pharmacometricians operating within our existing pharmaceutical, biotechnology, and chemical customers, as well as by new customers in other industries. However, if our strategies are not successfully implemented, our products and services may not achieve market acceptance or penetration in targeted new departments within our existing customers or in new industries. As a result, we may incur additional costs and expend additional resources without being able to sustain or increase revenue.
Consolidation within the pharmaceutical and biotechnology industries may continue to lead to fewer potential customers for our products and services.
A significant portion of our customer base consists of pharmaceutical and biotechnology companies. Consolidation within the pharmaceutical and biotechnology industries may result in fewer customers for our products and services. Although the industry consolidation that has taken place over the past 20 years has not prevented our business from growing to date, if one of the parties to a consolidation uses the products or services of our competitors, we may lose existing customers as a result of such consolidation.
Increasing competition and increasing costs within the pharmaceutical and biotechnology industries, drug development and services industry, and the life science market for modeling and simulation software and for cheminformatics products may affect the demand for our products and services, which may affect our results of operations and financial condition.
Our pharmaceutical and biotechnology customers’ demand for our products is impacted by continued demand for their products and by our customers’ research and development costs. Demand for our customers’ products could decline, and prices charged by our customers for their products may decline, as a result of increasing competition, including competition from companies manufacturing generic drugs. In addition, our customers’ expenses could continue to increase as a result of increasing costs of complying with government regulations and other factors. A decrease in demand for our customers’ products, pricing pressures associated with the sales of these products, and additional costs associated with product development, could cause our customers to reduce research and development expenditures. Although our products increase productivity and reduce costs in many areas, because our products and services depend on such research and development expenditures, our revenues may be significantly reduced.
Health care reform and restrictions on reimbursement may affect the pharmaceutical, biotechnology, and industrial chemical companies that purchase or license our products or services, which may affect our results of operations and financial condition.
The continuing efforts of government and third-party payers in the markets we serve to contain or reduce the cost of health care may reduce the profitability of pharmaceutical, biotechnology, and industrial chemical companies, causing them to reduce research and development expenditures. Because some of our products and services depend on such research and development expenditures, our revenues may be significantly reduced. We cannot predict what actions federal, state, or private payers for health care goods and services may take in response to any health care reform proposals or legislation.
| 17 |
We face strong competition in the life science market for modeling and simulation software and for cheminformatics products.
The market for our modeling and simulation software products for the life science market is intensely competitive. We currently face competition from other scientific software providers, larger technology and solutions companies, in-house development by our customers and academic and government institutions, and the open-source community. Some of our competitors and potential competitors have longer operating histories in certain segments of our industry than we do and could have greater financial, technical, marketing, research and development, and other resources. Many of our competitors offer products and services directed at more specific markets than those we target, enabling these competitors to focus a greater proportion of their efforts and resources on these markets. Some offerings that compete with our products are developed and made available at lower cost by government organizations and academic institutions, and these entities may be able to devote substantial resources to product development and also offer their products to users for little or no charge. We also face competition from open-source software initiatives, in which developers provide software and intellectual property free over the Internet. In addition, some of our customers spend significant internal resources in order to develop their own software. Moreover, we intend to leverage our scientific informatics platform in order to enable our customers to more effectively utilize the vast amounts of information stored in both their databases and public data sources in order to make informed scientific and business decisions during the research and development process. This strategy could lead to competition from much larger companies that provide general data storage and management software. There can be no assurance that our current or potential competitors will not develop products, services, or technologies that are comparable to, superior to, or render obsolete, the products, services, and technologies we offer. There can be no assurance that our competitors will not adapt more quickly than we to technological advances and customer demands, thereby increasing such competitors’ market share relative to ours. Any material decrease in demand for our technologies or services may have a material adverse effect on our business, financial condition, and results of operations.
We are subject to pricing pressures in some of the markets we serve.
The market for modeling and simulation products for the life science industry is intensely competitive. Although the average price of our software licenses has increased or remained relatively constant for fiscal years 2019, 2020, and 2021, we may experience a decline in the future. In response to increased competition and general adverse economic conditions in this market, we may be required to modify our pricing practices. Changes in our pricing model could adversely affect our revenues and earnings.
Our operations may be interrupted by the occurrence of a natural disaster or other catastrophic event at our primary facilities.
Our research and development operations and administrative functions are primarily conducted at our facilities in Lancaster, California, Buffalo, New York, Paris, France and Research Triangle Park, North Carolina. Although we have contingency plans in effect for natural disasters or other catastrophic events, the occurrence of such events could still disrupt our operations. For example, our Lancaster, California facility is located in a state that is particularly susceptible to earthquakes. Any natural disaster or catastrophic event in our facilities or the areas in which they are located could have a significant negative impact on our operations.
Our insurance coverage may not be sufficient to avoid material impact on our financial position or results of operations resulting from claims or liabilities against us, and we may not be able to obtain insurance coverage in the future.
We maintain insurance coverage for protection against many risks of liability. The extent of our insurance coverage is under continuous review and is modified as we deem it necessary. Despite this insurance, it is possible that claims or liabilities against us may have a material adverse impact on our financial position or results of operations. In addition, we may not be able to obtain any insurance coverage, or adequate insurance coverage, when our existing insurance coverage expires. For example, we do not carry earthquake insurance for our facilities in Lancaster, California, because we do not believe the costs of such insurance are reasonable in relation to the potential risk for our part of California.
| 18 |
Changes in government regulation or in practices relating to the pharmaceutical or biotechnology industries, including potential health care reform, could decrease the need for the services we provide.
Governmental agencies throughout the world, but particularly in the U.S., strictly regulate the drug development process. Our business involves helping pharmaceutical and biotechnology companies, among others, navigate the regulatory drug approval process. Accordingly, many regulations, and often new regulations, are expected to result in higher regulatory standards and often additional revenues for companies that service these industries. However, some changes in regulations, such as a relaxation in regulatory requirements or the introduction of streamlined or expedited drug approval procedures, or an increase in regulatory requirements that we have difficulty satisfying or that make our services less competitive, could eliminate or substantially reduce the demand for our services.
Any negative commentaries made by any regulatory agencies or any failure by us to comply with applicable regulations and related guidance could harm our reputation and operating results, and compliance with new regulations and guidance may result in additional costs.
Any negative commentaries made by any regulatory agencies or any failure on our part to comply with applicable regulations could result in the termination of ongoing research or the disqualification of data for submission to regulatory authorities. This could harm our reputation, our prospects for future work, and our operating results. If our operations are found to violate any applicable law or other governmental regulations, we might be subject to civil and criminal penalties, damages, and fines. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business, and damage our reputation.
Our sales cycle is lengthy and customers may delay entering into contracts or decide not to adopt our products or solutions after we have expended significant time and resources and supported evaluation by them of our technology, which could result in delays in recognizing revenue and negatively impact our results of operations.
On-going negotiations and evaluation projects for new products, with new customers or in new markets may not result in significant revenues for us if we are unable to close new engagements on terms favorable to us, in a timely manner, or at all. Unexpected delays in our sales cycle could cause our revenues to fall short of expectations. Further, the timing and length of negotiations required to enter into agreements with our customers and the ultimate enforcement of complex negotiated contractual provisions as we intended is difficult to predict. If we do not successfully negotiate certain key complex contractual provisions, there are disputes regarding such provisions, or they are not enforceable as we intended, our revenues and results of operations would suffer. Further, if we were to incur significant effort and then fail to enter into final contracts with prospective customers, or if a contract is terminated earlier than expected, our revenues and results of operations could suffer.
Many of our contracts are fixed-price and may be delayed or terminated or reduced in scope for reasons beyond our control, or we may underprice or overrun cost estimates with these contracts, potentially resulting in financial losses.
Many of our contracts provide for services on a fixed-price or fee-for-service with a cap basis and, accordingly, we bear the financial risk if we initially underprice our contracts or otherwise overrun our cost estimates. In addition, these contracts may be terminated or reduced in scope either immediately or upon notice. Cancellations may occur for a variety of reasons, and often at the discretion of the client. The loss, reduction in scope, or delay of a large contract or the loss or delay of multiple contracts could materially adversely affect our business, although our contracts frequently entitle us to receive the costs of winding down the terminated projects, as well as all fees earned by us up to the time of termination. Some contracts also entitle us to a predetermined termination fee and irrevocably committed costs/expenses.
| 19 |
We could experience a breach of the confidentiality of the information we hold or of the security of our computer systems.
We operate large and complex computer systems that contain significant amounts of client data. As a routine element of our business, we collect, analyze, and retain substantial amounts of data pertaining to the clinical study data analysis we conduct for our clients. Unauthorized third parties could attempt to gain entry to such computer systems for the purpose of stealing data or disrupting the systems. We believe that we have taken appropriate measures to protect them from intrusion, and we continue to improve and enhance our systems in this regard, but in the event that our efforts are unsuccessful, we could suffer significant harm. Our contracts with our clients typically contain provisions that require us to keep confidential the information generated from these studies. In the event the confidentiality of such information was compromised, we could suffer significant harm.
Impairment of goodwill or intangible assets may adversely impact future results of operations.
We have intangible assets, including goodwill, capitalized computer software development costs, intellectual property, and other intangible assets, on our balance sheet due to our acquisitions of businesses. The initial identification and valuation of these intangible assets and the determination of the estimated useful lives at the time of acquisition involve use of management judgments and estimates. These estimates are based on, among other factors, input from accredited valuation consultants, reviews of projected future income cash flows, and statutory regulations. The use of alternative estimates and assumptions might have increased or decreased the estimated fair value of our goodwill and intangible assets that could potentially result in a different impact to our results of operations. If the future growth and operating results of our business are not as strong as anticipated and/or our market capitalization declines, this could impact the assumptions used in calculating the fair value of goodwill or intangibles. To the extent goodwill or intangibles are impaired, their carrying value will be written down to its implied fair value and a charge will be made to our income from continuing operations. Such an impairment charge could materially and adversely affect our operating results. As of August 31, 2021, and 2020, the carrying amount of goodwill and intangibles was $37.5 and $37.9 million, respectively, on our consolidated balance sheet.
Certain Risks Related to Our Operations
Software defects or malfunctions in our products could hurt our reputation among our customers, result in delayed or lost revenue, and expose us to liability.
Our business and the level of customer acceptance of our products depend upon the continuous, effective, and reliable operation of our software and related tools and functions. To the extent that defects cause our software to malfunction and our customers’ use of our products is interrupted, our reputation could suffer and our revenue could decline or be delayed while such defects are remedied. We may also be subject to liability for the defects and malfunctions of third-party technology partners and others with whom our products and services are integrated.
Delays in the release of new or enhanced products or services or undetected errors in our products or services may result in increased cost to us, delayed market acceptance of our products, and delayed or lost revenue.
To achieve market acceptance, new or enhanced products or services can require long development and testing periods, which may result in delays in scheduled introduction. Any delays in the release schedule for new or enhanced products or services may delay market acceptance of these products or services and may result in delays in new customer orders for these new or enhanced products or services or the loss of customer orders. In addition, new or enhanced products or services may contain a number of undetected errors or “bugs” when they are first released. Although we extensively test each new or enhanced software product or service before it is released to the market, there can be no assurance that significant errors will not be found in existing or future releases. As a result, in the months following the introduction of certain releases, we may need to devote significant resources to correct these errors. There can be no assurance, however, that all of these errors can be corrected.
| 20 |
We are subject to risks associated with the operation of a global business.
We derive a significant portion of our total revenue from our operations in international markets. During the years ended August 31, 2021, 2020 and 2019, 31%, 29% and 34% respectively, of our total revenue was derived from our international operations. Our global business may be affected by local economic conditions, including inflation, recession, and currency exchange rate fluctuations. In addition, political and economic changes, including international conflicts and terrorist acts, throughout the world may interfere with our or our customers’ activities in particular locations and result in a material adverse effect on our business, financial condition, and operating results. Potential trade restrictions, exchange controls, adverse tax consequences, and legal restrictions may affect the repatriation of funds into the U.S. Also, we could be subject to unexpected changes in regulatory requirements, the continued global spread and impact of the COVID-19 pandemic, the difficulties of compliance with a wide variety of foreign laws and regulations, potentially negative consequences from changes in or interpretations of U.S. and foreign tax laws, import and export licensing requirements, and longer accounts receivable cycles in certain foreign countries. These risks, individually or in the aggregate, could have an adverse effect on our results of operations and financial condition. For example, we are subject to compliance with the U.S. Foreign Corrupt Practices Act and similar anti-bribery laws, which generally prohibit companies and their intermediaries from making improper payments to foreign government officials for the purpose of obtaining or retaining business. While our employees, distributors, and agents are required to comply with these laws, we cannot be sure that our internal policies and procedures will always protect us from violations of these laws despite our commitment to legal compliance and corporate ethics. The occurrence or allegation of these types of risks may adversely affect our business, performance, prospects, value, financial condition, and results of operations.
The drug discovery and development services industry is highly competitive.
Our clinical pharmacology division often competes for business not only with other clinical research organization (“CROs”), but also with internal discovery and development departments within our larger clients, who may have greater resources than ours. We also compete with universities and teaching hospitals for outsourced services. We compete based on a variety of factors, including without limitation:
| · | reputation for on-time quality performance; | |
| · | reputation for regulatory compliance; | |
| · | expertise and experience in multiple specialized areas; | |
| · | scope and breadth of service and product offerings across the drug discovery and development spectrum; | |
| · | ability to provide flexible and customized solutions to support our clients’ drug discovery and development needs; | |
| · | price/value; | |
| · | technological expertise and efficient drug development processes; | |
| · | financial stability; | |
| · | accessibility of client data through secure portals; and | |
| · | ability to acquire, process, analyze, and report data in an accurate manner. |
| 21 |
If we do not compete successfully, our business could suffer. Increased competition could lead to price and other concessions that might adversely affect our operating results. The drug discovery and development services industry has continued to see a trend towards consolidation, particularly among biotechnology companies, who are acquisition targets for each other and for larger pharmaceutical companies. If this trend continues, it is likely to produce more competition among the larger companies and CROs generally, with respect to both clients and acquisition candidates. In addition, while there are substantial barriers to entry for large, global competitors with broad-based services, small, specialized entities considering entering the CRO industry will continue to find lower barriers to entry, and private equity firms may determine that there are opportunities to acquire and consolidate these companies, thus further increasing possible competition. More generally, our competitors or others might develop technologies, services, or products that are more effective or commercially attractive than our current or future technologies, services, or products, or that render our technologies, services, or products less competitive or obsolete. If competitors introduce superior technologies, services, or products and we cannot make enhancements to ours to remain competitive, our competitive position, and in turn our business, revenue, and financial condition, would be materially and adversely affected. In the aggregate, these competitive pressures may affect the attractiveness of our technologies, services, or products and could adversely affect our financial results.
Changes in applicable U.S. and international tax laws or regulations and the resolution of tax disputes could negatively affect our financial results.
We are subject to income taxes, as well as non-income-based taxes, in both the U.S. and various foreign jurisdictions in which we do business. Significant judgment is required in determining our worldwide provision for income taxes and other tax liabilities. Changes in tax laws or tax rulings may have a significant adverse impact on our effective tax rate. For example, the U.S. and many countries where we do business are actively considering or have recently enacted changes in relevant tax, accounting and other laws, regulations and interpretations. Recently, the Biden Administration committed to increasing the corporate income tax rate, and to increasing the tax rate applied to profits earned outside the U.S. If enacted, the impact of these potential new rules could be material to our tax provision and value of deferred tax assets and liabilities.
Further, in the ordinary course of a global business, there are many intercompany transactions and calculations where the ultimate tax determination could change if tax laws or tax rulings were to be modified. We are also subject to non-income-based taxes, such as payroll, sales, use, value-added, net worth, property and goods and services taxes, in both the U.S. and various foreign jurisdictions. Although we believe that our income and non-income-based tax estimates are appropriate, there is no assurance that the final determination of tax audits or tax disputes will not be different from what is reflected in our historical income tax provisions and accruals.
Given the unpredictability of possible further changes to the U.S. or foreign tax laws and regulations and their potential interdependency, it is very difficult to predict the cumulative effect of such tax laws and regulations on our results of operations and cash flow, but such laws and regulations (and changes thereto) could adversely impact our financial results.
Contract research services create a risk of liability.
As a CRO, we face a range of potential liabilities which may include:
| · | Errors or omissions in reporting of study detail in preclinical studies that may lead to inaccurate reports, which may undermine the usefulness of a study or data from the study, or which may potentially advance studies absent the necessary support or inhibit studies from proceeding to the next level of testing; and | |
| · | Risks associated with our possible failure to properly care for our clients’ property, such as research models, records, work in progress, or other archived materials. |
Contractual risk transfer indemnifications generally do not protect us against liability arising from certain of our own actions, such as negligence or misconduct. We could be materially and adversely affected if we are required to pay damages or bear the costs of defending any claim that is outside any contractual indemnification provision, or if a party does not fulfill its indemnification obligations, or the damage is beyond the scope or level of insurance coverage. We also often contractually indemnify our clients (subject to a limitation of liability), similar to the way they indemnify us, and we may be materially adversely affected if we have to fulfill our indemnity obligations. Furthermore, there can be no assurance that we nor a party required to indemnify us will be able to maintain such insurance coverage (either at all or on terms acceptable to us).
| 22 |
Upgrading our software could result in implementation issues and business disruptions.
We update our software on a regular basis and are continually in the process of refactoring our software programs. In doing so, we face the possibility that existing users will find the software unacceptable, or new users may not be as interested as they have been in the past versions. Translation errors might introduce new software bugs that will not be caught.
The drug discovery and development industry has a history of patent and other intellectual property litigation, and we might be involved in costly intellectual property lawsuits.
The drug discovery and development industry has a history of patent and other intellectual property litigation and these lawsuits will likely continue. Accordingly, we face potential patent infringement suits by companies that have patents for similar products and methods used in business or other suits alleging infringement of their intellectual property rights. Legal proceedings relating to intellectual property could be expensive, take significant time, and divert management’s attention from other business concerns, whether we win or lose. If we do not prevail in an infringement lawsuit brought against us, we might have to pay substantial damages, including treble damages, and we could be required to stop the infringing activity or obtain a license to use technology on unfavorable terms.
We may not be able to successfully develop and market new services and products.
We may seek to develop and market new services and products that complement or expand our existing business or service offerings. We cannot guarantee that we will be able to identify new technologies of interest to our customers. Even if we are able to identify new technologies of interest, we may not be able to negotiate license agreements on acceptable terms, or at all. If we are unable to develop new services and products and/or create demand for those newly developed services and products, our future business, results of operations, financial condition, and cash flows could be adversely affected.
Ability to incur debt could adversely affect our business and growth prospects.
On March 31, 2020 we established a line of credit with a bank in the amount of $3,500,000 and, to date, we have not accessed the line. Prior to the establishment of the line we have not had any borrowed debt and have no need to do so to fund normal operations in the foreseeable future. Should circumstances require us to incur additional debt and a lender could not be found to provide that debt, this could have a significant adverse effect on our business, including making it more difficult for us to obtain financing on favorable terms, limiting our ability to capitalize on significant business opportunities, and making us more vulnerable to rising interest rates.
We depend on key personnel and may not be able to retain these employees or recruit additional qualified personnel, which could harm our business.
Our success depends to a significant extent on the continued services of our senior management and other members of management. We have employment agreements with our CEO, CFO and division presidents that range from one to three years. If our CEO, CFO, division presidents, or other members of senior management do not continue in their present positions, our business may suffer. Because of the specialized scientific nature of our business, we are highly dependent upon attracting and retaining qualified scientific and technical and managerial personnel. While we have a strong record of employee retention, there is still significant competition for qualified personnel in the software, pharmaceutical, and biotechnology fields. Therefore, we may not be able to attract and retain the qualified personnel necessary for the development of our business. The loss of the services of existing personnel, as well as the failure to recruit additional key scientific, technical, and managerial personnel in a timely manner, could harm our business.
| 23 |
If we are not successful in selecting and integrating the businesses and technologies we acquire, or in managing our current and future divestitures, our business may suffer.
Over the years, we have expanded our business through acquisitions. We continue to search to acquire businesses and technologies and form strategic alliances. However, businesses and technologies may not be available on terms and conditions we find acceptable. We risk spending time and money investigating and negotiating with potential acquisition or alliance partners, but not completing transactions. Even if completed, acquisitions and alliances involve numerous risks which may include: difficulties in achieving business and continuing financial success; difficulties and expenses incurred in assimilating and integrating operations, services, products, technologies, or pre-existing relationships with our customers, distributors, and suppliers; challenges with developing and operating new businesses, including those which are materially different from our existing businesses and which may require the development or acquisition of new internal capabilities and expertise; challenges of maintaining staffing at the acquired entities, including loss of key employees; potential losses resulting from undiscovered liabilities of acquired companies that are not covered by the indemnification we may obtain from the seller(s); the presence or absence of adequate internal controls and/or significant fraud in the financial systems of acquired companies; diversion of management’s attention from other business concerns; acquisitions could be dilutive to earnings, or in the event of acquisitions made through the issuance of our common stock to the shareholders of the acquired company, dilutive to the percentage of ownership of our existing shareholders; new technologies and products may be developed which cause businesses or assets we acquire to become less valuable; and risks that disagreements or disputes with prior owners of an acquired business, technology, service, or product may result in litigation expenses and distribution of our management’s attention. In the event that an acquired business or technology or an alliance does not meet our expectations, our results of operations may be adversely affected.
Some of the same risks exist when we decide to sell a business, site, or product line. In addition, divestitures could involve additional risks, including, without limitation, the following: difficulties in the separation of operations, services, products, and personnel; and the need to agree to retain or assume certain current or future liabilities in order to complete the divestiture. We evaluate the performance and strategic fit of our businesses. These and any divestitures may result in significant write-offs, including those related to goodwill and other intangible assets, which could have an adverse effect on our results of operations and financial condition. In addition, we may encounter difficulty in finding buyers or alternative exit strategies at acceptable prices and terms and in a timely manner. We may not be successful in managing these or any other significant risks that we encounter in divesting a business, site, or product line, and as a result, we may not achieve some or all of the expected benefits of the divestitures.
Our quarterly and annual operating results fluctuate and may continue to fluctuate in the future, and if we fail to meet the expectations of analysts or investors, our stock price and the value of your investment could decline substantially.
We believe that operating results for any particular quarter are not necessarily a meaningful indication of future results. Nonetheless, fluctuations in our quarterly operating results could negatively affect the market price of our common stock. Our results of operations in any quarter or annual period have varied in the past and may vary from quarter to quarter or year to year. Our results of operations are influenced by various factors, many of which are out of our control, including without limitation:
| · | changes in the general global economy; | |
| · | the number and scope of ongoing client engagements; the commencement, postponement, delay, progress, completion, or cancellation of client contracts in the quarter; | |
| · | changes in customer budget cycles; | |
| · | the number and scope of ongoing client engagements; | |
| · | the commencement, postponement, delay, progress, completion, or cancellation of client contracts in the quarter; | |
| · | changes in the mix of our products and services; |
| 24 |
| · | competitive pricing pressures; | |
| · | the extent of cost overruns; | |
| · | buying patterns of our clients; | |
| · | budget cycles of our clients; | |
| · | the effect of potential acquisitions and consequent integration; | |
| · | the timing of new product releases by us or our competitors; | |
| · | general economic factors, including factors relating to disruptions in the world credit and equity markets and the related impact on our customers’ access to capital; | |
| · | changes in tax laws, rules, regulations, and tax rates in the locations in which we operate; | |
| · | the timing and charges associated with completed acquisitions and other events; | |
| · | the financial performance of our investments; and | |
| · | exchange rate fluctuations. |
We derive a significant percentage of our revenues from a concentrated group of customers and the loss of more than one of our major customers could materially and adversely affect our business, results of operations or financial condition.
Three customers accounted for 11%, 4% and 3%, respectively, of revenue for fiscal year 2021. Three customers accounted for 9%, 7% (a dealer account in Japan representing various customers), and 7%, respectively, of revenues for fiscal year 2020. Three customers accounted for 8%, 8% and 7% (a dealer account in Japan representing various customers), respectively, of revenues for fiscal year 2019. The loss of any of our major customers could have a material adverse effect on our results of operations and financial condition. We may not be able to maintain our customer relationships, and our customers may delay payment under, or fail to renew, their agreements with us, which could adversely affect our business, results of operations, or financial condition. Any reduction in the amount of revenues that we derive from these customers, without an offsetting increase in new revenues to other customers, could have a material adverse effect on our operating results. A significant change in the liquidity or financial position of our customers could also have a material adverse effect on the collectability of our accounts receivable, our liquidity, and our future operating results.
We conduct business outside the U.S., which exposes us to foreign currency exchange rate risk, amongst other risk, and could have a negative impact on our financial results.
We operate on a global basis. In the three years ended August 31, 2021, 2020 and 2019, we had revenues of $4.8 million, $5.0 million, and $4.1 million, respectively, denominated in foreign currency in certain Asian and European markets. We expanded our operations in Europe in 2020 with the addition of Lixoft in Paris, France.
As we continue to increase our international operations, our revenues and expenditures in foreign currencies are expected to become more material and subject to greater foreign currency exchange rate fluctuations. Also, our foreign distributors typically sell our products in local currency, which impacts the price to foreign consumers. Our subsidiary operates with their local currency as their functional currency. Future foreign currency exchange rate fluctuations and global credit markets may cause changes in the U.S. dollar value of our purchases or sales and materially affect our revenues, profit margins, and results of operations, when converted to U.S. dollars. Changes in the value of the U.S. dollar relative to other currencies could result in material foreign currency exchange rate fluctuations and, as a result, our net earnings could be materially adversely affected.
| 25 |
As we continue to expand international operations and increase purchases and sales in foreign currencies, we may utilize derivative instruments, as needed, to hedge our foreign currency exchange rate risk. Our hedging strategies will depend on our forecasts of revenues, expenses, and cash flows, which are inherently subject to inaccuracies. Foreign currency exchange rate hedges, transactions, re-measurements, or translations could materially impact our consolidated financial statements.
A significant portion of our operating expenses is relatively fixed and planned expenditures are based in part on expectations regarding future revenues.
Accordingly, unexpected revenue shortfalls may decrease our gross margins and could cause significant changes in our operating results from year to year. As a result, in future quarters, our operating results could fall below the expectations of securities analysts or investors, in which event our stock price would likely decrease.
If our customers cancel their contracts or terminate or delay their clinical trials, we may lose or delay revenues and our business may be harmed.
Certain of our customer contracts are subject to cancellation by our customers at any time with limited notice. Customers engaged in clinical trials may terminate or delay a clinical trial for various reasons, including the failure of the tested product to satisfy safety or efficacy requirements, unexpected or undesired clinical results, decisions to deemphasize a particular product or forgo a particular clinical trial, decisions to downsize clinical development programs, insufficient patient enrollment or investigator recruitment, and production problems resulting in shortages of required clinical supplies. Any termination or delay in the clinical trials would likely result in a consequential delay or termination in those customers’ service contracts. We have experienced terminations and delays of our customer service contracts in the past (although no such past terminations have had a significant impact on our results of operations) and we expect to experience additional terminations and delays in the future. The termination of single-study arrangements could result in decreased revenues and the delay of our customers’ clinical trials could result in delayed professional services revenues, which could materially harm our business.
If our security is breached, our business could be disrupted, our operating results could be harmed, and customers could be deterred from using our products and services.
Our business relies on the secure electronic transmission, storage, and hosting of sensitive information, including clinical data, financial information, and other sensitive information relating to our customers, company, and workforce. As a result, we face some risk of a deliberate or unintentional incident involving unauthorized access to our computer systems (including, among other methods, cyber- attacks or social engineering) that could result in misappropriation or loss of assets or sensitive information, data corruption, or other disruption of business operations. In light of this risk, we have devoted significant resources to protecting and maintaining the confidentiality of our information, including implementing security and privacy programs and controls, training our workforce, and implementing new technology. We have no guarantee that these programs and controls will be adequate to prevent all possible security threats. We believe that any compromise of our electronic systems, including the unauthorized access, use, or disclosure of sensitive information, or a significant disruption of our computing assets and networks, would adversely affect our reputation and our ability to fulfill contractual obligations, and would require us to devote significant financial and other resources to mitigate such problems, and could increase our future cyber security costs. Moreover, unauthorized access, use, or disclosure of such sensitive information could result in contractual or other liability. In addition, any real or perceived compromise of our security or disclosure of sensitive information may result in lost revenues by deterring customers from using or purchasing our products and services in the future or prompting them to use competing service providers.
Any failure by us to properly protect customer data we possess or are deemed to possess, in connection with the conduct of clinical trials, could subject us to significant liability.
Our customers use our solutions to collect, manage, and report information in connection with the conduct of clinical trials. This information may be considered our customers’ proprietary information. Since we receive and process our customers’ data from customers utilizing our hosted solutions, there is a risk that we could be liable if there were a breach of any obligation to a protected person under contract, standard of practice, or regulatory requirement. If we fail to properly protect our customers’ data that is in our possession or deemed to be in our possession, we could be subjected to significant liability and our reputation would be harmed.
| 26 |
We rely upon a single internal hosting facility and Amazon Web Services to deliver certain solutions to our customers and any disruption of or interference with our hosting systems, operations, or use of the Amazon Web Services could harm our business and results of operations.
Substantially all of the computer hardware necessary to provide our Cognigen solutions to our customers is located at our internal hosting facility in Buffalo, New York. In addition to our dedicated hosting facility, we utilize third-party cloud computing services from Amazon Web Services (“AWS”) to help us efficiently scale our cloud-based solutions and provide training. Because we cannot easily switch our AWS-serviced operations to another cloud provider, any disruption of or interference with our use of AWS would impact our operations, and our business would be adversely impacted. Our systems and operations or those of AWS could suffer damage or interruption from human error, fire, flood, power loss, telecommunications failure, break-ins, terrorist attacks, acts of war, and similar events. The occurrence of a natural disaster, an act of terrorism or other unanticipated problems at our or AWS’ hosting facilities could result in lengthy interruptions in our service. Although we and AWS maintain backup facilities and disaster recovery services in the event of a system failure, these may be insufficient or fail. Any system failure, including network, software, or hardware failure, that causes an interruption in our Buffalo data center or our use of AWS or that causes a decrease in responsiveness of our cloud-based solutions, could damage our reputation and cause us to lose customers, which could harm our business and results of operations. Our business may be harmed if our customers and potential customers believe our service is unreliable.
Defects or errors in our software applications could harm our reputation, result in significant cost to us and impair our ability to market our solutions.
Our software applications are inherently complex and may contain defects or errors, some of which may be material. Errors may result from our own technology or from the interface of our cloud-based solutions with legacy systems and data, which we did not develop. The risk of errors is particularly significant when a new product is first introduced or when new versions or enhancements of existing products are released. The likelihood of errors is increased when we do more frequent releases of new products and enhancements of existing products. We have, from time to time, found defects in our solutions. Although these past defects have not resulted in any litigation against us to date, we have invested significant capital, technical, managerial, and other resources to investigate and correct these past defects and we have needed to divert these resources from other development efforts. In addition, material performance problems or defects in our solutions may arise in the future. Material defects in our cloud-based solutions could result in a reduction in revenues, delay in market acceptance of our solutions, or credits or refunds to our customers. In addition, such defects may lead to the loss of existing customers and difficulty in attracting new customers, diversion of development resources, or harm to our reputation. Correction of defects or errors could prove to be impossible or impractical. The costs incurred in correcting any defects or errors or in responding to resulting claims or liability may be substantial and could adversely affect our operating results.
If we are not able to reliably meet our data storage and management requirements, or if we experience any failure or interruption in the delivery of our services over the Internet, customer satisfaction and our reputation could be harmed and customer contracts may be terminated.
As part of our current business model, we deliver our software over the Internet and store and manage hundreds of terabytes of data for our customers, resulting in substantial information technology infrastructure and ongoing technological challenges, which we expect to continue to increase over time. If we do not reliably meet these data storage and management requirements, or if we experience any failure or interruption in the delivery of our services over the Internet, customer satisfaction and our reputation could be harmed, leading to reduced revenues and increased expenses. Our hosting services are subject to service-level agreements and, in the event that we fail to meet guaranteed service or performance levels, we could be subject to customer credits or termination of these customer contracts. If the cost of meeting these data storage and management requirements increases, our results of operations could be harmed.
| 27 |
Some of our software solutions and services utilize open source software, and any failure to comply with the terms of one or more of these open source licenses could adversely affect our business.
Some of our software solutions utilize software covered by open-source licenses. Open-source software is typically freely accessible, usable and modifiable, and is used by our development team in an effort to reduce development costs and speed up the development process. Certain open-source software licenses require a user who intends to distribute the open-source software as a component of the user’s software to disclose publicly part or all of the source code to the user’s software. In addition, certain open-source software licenses require the user of such software to make any derivative works of the open-source code available to others on unfavorable terms or at no cost. This can subject previously proprietary software to open-source license terms. While we monitor the use of all open-source software in our products, processes, and technology and try to ensure that no open-source software is used in such a way as to require us to disclose or make available the source code to the related product or solution, such use could inadvertently occur. This could harm our intellectual property position and have a material adverse effect on our business.
We may be unable to adequately enforce or defend our ownership and use of our intellectual property and other proprietary rights.
Our success is heavily dependent upon our intellectual property and other proprietary rights. We rely upon a combination of trademark, trade secret, copyright, patent, and unfair competition laws, as well as license and access agreements and other contractual provisions, to protect our intellectual property and other proprietary rights. In addition, we attempt to protect our intellectual property and proprietary information by requiring certain of our employees and consultants to enter into confidentiality, noncompetition, and assignment-of-inventions agreements. The steps we take to protect our intellectual property rights may not be adequate to prevent misappropriation of our technology by third parties, or may not be adequate under the laws of some foreign countries, which may not protect our intellectual property rights to the same extent as do the laws of the United States.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. This could make it difficult for us to stop infringement or the misappropriation of our intellectual property rights.
Our attempts to protect our intellectual property may be challenged by others or invalidated through administrative process or litigation, and agreement terms that address noncompetition are difficult to enforce in many jurisdictions and may not be enforceable in any particular case. In addition, there remains the possibility that others will “reverse engineer” our products in order to introduce competing products, or that others will develop competing technology independently. If we resort to legal proceedings to enforce our intellectual property rights or to determine the validity and scope of the intellectual property or other proprietary rights of others, the proceedings could be burdensome and expensive, even if we were to prevail. The failure to adequately protect our intellectual property and other proprietary rights may have a material adverse effect on our business, results of operations or financial condition.
Current and future litigation against us, which may arise in the ordinary course of our business, could be costly and time-consuming to defend.
We are subject to claims that arise in the ordinary course of business, such as claims brought by our customers in connection with commercial disputes and employment claims made by our current or former employees. Third parties may in the future assert intellectual property rights to technologies that are important to our business and demand back royalties or demand that we license their technology. Litigation may result in substantial costs and may divert management’s attention and resources, which may seriously harm our business, overall financial condition, and operating results. Insurance may not cover such claims, may not be sufficient for one or more such claims, and may not continue to be available on terms acceptable to us. A claim brought against us that is uninsured or underinsured could result in unanticipated costs, negatively affecting our business, results of operations, and financial condition.
| 28 |
We could incur substantial costs resulting from product liability claims relating to our products or services or our customers’ use of our products or services.
Any failure or errors in a customer’s clinical trial caused or allegedly caused by our products or services could result in a claim for substantial damages against us by our customers or the clinical trial participants, regardless of our responsibility for the failure. Although we are generally entitled to indemnification under our customer contracts against claims brought against us by third parties arising out of our customers’ use of our products, we might find ourselves entangled in lawsuits against us that, even if unsuccessful, may divert our resources and energy and adversely affect our business. Further, in the event we seek indemnification from a customer, a court may not enforce our indemnification right if the customer challenges it or the customer may not be able to fund any amounts for indemnification owed to us. In addition, our existing insurance coverage may not continue to be available on reasonable terms or may not be available in amounts sufficient to cover one or more large claims, or the insurer may disclaim coverage as to any future claim.
Our business depends on the clinical trial market, and a downturn in this market could cause our revenues to decrease.
Our business depends on clinical trials conducted or sponsored by pharmaceutical, biotechnology, and medical device companies, CROs, and other entities. Our revenues may decline as a result of conditions affecting these industries, including general economic downturns, increased consolidation, decreased competition, or fewer products under development. Other developments that may affect these industries and harm our operating results include product liability claims, changes in government regulation, changes in governmental price controls or third-party reimbursement practices, and changes in medical practices. Disruptions in the world credit and equity markets may also result in a global downturn in spending on research and development and clinical trials and may impact our customers’ access to capital and their ability to pay for our solutions. Any decrease in research and development expenditures or in the size, scope, or frequency of clinical trials could materially adversely affect our business, results of operations, or financial condition.
As a public company, we are obligated to maintain proper and effective internal control over financial reporting. As our business expands both organically and through acquisitions, we may be unable to effectively adapt our current systems to our changing business needs and may fail to develop and maintain an effective system of disclosure controls and internal control over financial reporting which could impair our ability to produce timely and accurate financial statements or comply with applicable laws and regulations.
As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (the “Dodd-Frank Act”), and other applicable securities rules and regulations. Compliance with these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time consuming, or costly, and increase demand on our systems and resources. The Exchange Act requires, among other things, that we file annual, quarterly, and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. As a company, we continually review and evaluate the adequacy of our disclosure controls and procedures and internal controls over financial reporting for deficiencies and improvements.
As we expand our operations through acquisitions and organic growth, our current systems for disclosure controls and procedures and internal control over financial reporting may be inadequate to meet our growing and changing business. Accordingly, we may require significant resources and management oversight to maintain and, if necessary, improve our disclosure controls and procedures and internal control over financial reporting. As a result, management’s attention may be diverted from other business concerns, which could adversely affect our business and operating results. In addition, we may need to hire more employees in the future or engage outside consultants with respect to developing and maintaining our disclosure controls and internal control over financial reporting, which would increase our costs and expenses.
| 29 |
In addition, as a public company, we are required, pursuant to Section 404 of the Sarbanes-Oxley Act to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. Effective internal control over financial reporting is necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. As a result of the growth of our business both organically and through acquisitions, we may fail to implement required new or improved controls, or experience difficulties in their implementation, which may cause us to not meet our reporting obligations. If we or our independent registered public accounting firm were to identify a material weakness, if we are unable to assert that our internal control over financial reporting is effective, we could lose investor confidence in the accuracy and completeness of our financial reports, which could cause the price of our common stock to decline, and we may be subject to investigation by the SEC.
As a public company, we may incur significant administrative workload and expenses in connection with new and changing compliance requirements.
As a public company with common stock listed on The Nasdaq Global Select Market, we must comply with various laws, regulations and requirements. New laws and regulations, as well as changes to existing laws and regulations affecting public companies, including the provisions of the Sarbanes-Oxley Act, the Dodd-Frank Act, and rules adopted by the SEC and by the Nasdaq Global Select Market, may result in increased general and administrative expenses and a diversion of management’s time and attention as we respond to new requirements.
Certain Risks Related to Ownership of Our Common Stock
We have been paying quarterly dividends on shares of our common stock, and although there has been a consistent track record of paying these dividends, the Board of Directors may suspend the dividend, and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
Should the Board of Directors suspend the dividend and decide to use those funds to invest more into our business, you may not receive any dividends on your investment in our common stock for the foreseeable future and the success of an investment in shares of our common stock will depend upon any future appreciation in its value. Shares of our common stock may depreciate in value or may not appreciate in value.
If our operating and financial performance in any given period does not meet any guidance that we provide to the public, the market price of our common stock may decline.
We may, but are not obligated to, provide public guidance on our expected operating and financial results for future periods. Any such guidance will be comprised of forward-looking statements subject to the risks and uncertainties described in this prospectus and in our other public filings and public statements. Our actual results may not always be in line with or exceed any guidance we have provided, especially in times of economic uncertainty. If, in the future, our operating or financial results for a particular period do not meet any guidance we provide or the expectations of investment analysts, or if we reduce our guidance for future periods, the market price of our common stock may decline. Even if we do issue public guidance, there can be no assurance that we will continue to do so in the future.
| 30 |
The price of our common stock may fluctuate significantly, and investors could lose all or part of their investments.
Shares of our common stock were sold in our initial public offering (“IPO”) in 1996 at a price of $1.25 per share (on a post-split basis), and our common stock has subsequently traded as high as $90.92 and as low as $0.38 from our IPO through August 31, 2021. However, an active, liquid, and orderly market for our common stock on the Nasdaq Global Select Market or otherwise may not be sustained, which could depress the trading price of our common stock. The trading price of our common stock may be subject to wide fluctuations in response to various factors, some of which are beyond our control, including without limitation:
| · | our quarterly or annual earnings or those of other companies in our industry; | |
| · | announcements by us or our competitors of significant contracts or acquisitions; | |
| · | changes in accounting standards, policies, guidance, interpretations, or principles; | |
| · | general economic and stock market conditions, including disruptions in the world credit and equity markets; | |
| · | the failure of securities analysts to cover our common stock or changes in financial estimates by analysts; | |
| · | future sales of our common stock; and | |
| · | the other factors described in these “Risk Factors.” |
In recent years, the stock market in general, and the market for technology-related companies in particular, has experienced wide price and volume fluctuations. This volatility has had a significant impact on the market price of securities issued by many companies, including companies in our industry. The price of our common stock could fluctuate based upon factors that have little to do with our performance, and these fluctuations could materially reduce our stock price.
In the past, some companies, including companies in our industry, have had volatile market prices for their securities and have had securities class action suits filed against them. The filing of a lawsuit against us, regardless of the outcome, could have a material adverse effect on our business, financial condition, and results of operations, as it could result in substantial legal costs and a diversion of our management’s attention and resources.
The price of our common stock may be volatile, and our stockholders may not be able to resell shares of our common stock at or above the price they paid.
The trading price of our common stock is volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. Factors that could cause volatility in the market price of our common stock include, but are not limited to:
| · | achievement of expected software product and consulting service revenues and profitability, including the effects of seasonality on our results of operations, as well as adjustments to our revenues forecasts; |
| · | the ongoing COVID-19 pandemic, see “—Certain Risks Related to our Business—Our business is subject to risks arising from epidemic diseases, such as the recent outbreak of the COVID-19 illness.” |
| · | announcements of new products by us or our competitors; |
| · | announcements or developments in any intellectual property infringement actions in which we may become involved; |
| 31 |
| · | our operating results; |
| · | results from, or any delays in, clinical trial programs of our clients and their need for our services; |
| · | changes or developments in laws or regulations applicable to our products; | |
| · |
consolidation within the pharmaceutical and biotechnology industries leading to fewer potential customers for our products and services; | |
| · | delays in the release of new or enhanced products or services or undetected errors in our products or services may result in increased cost to us, delayed market acceptance of our products, and delayed or lost revenue; | |
| · | adverse actions taken by regulatory agencies with respect to our clinical trials, manufacturing supply chain, or sales and marketing activities; |
| · | the success of our efforts to acquire or develop additional products; |
| · | announcements concerning our competitors or the pharmaceutical industry in general; |
| · | actual or anticipated fluctuations in our operating results; |
| · | FDA or other U.S. or foreign regulatory actions affecting us or our industry or other healthcare reform measures in the United States; |
| · | changes in financial estimates or recommendations by securities analysts; |
| · | trading volume of our common stock; |
| · | sales of our common stock by us, our executive officers and directors, or our stockholders in the future; |
| · | general economic and market conditions and overall fluctuations in the United States equity markets, including as a result of volatility related to the recent coronavirus outbreak and related health concerns; and |
| · | the loss of any of our key scientific or management personnel. |
Broad market fluctuations may adversely affect the trading price or liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our management would be diverted from the operation of our business, which could seriously harm our financial position. Any adverse determination in litigation could also subject us to significant liabilities.
If securities or industry analysts issue an adverse or misleading opinion regarding our stock, or our inclusion in the S&P 600 discontinues, our stock price and trading volume could decline.
The trading market for our common stock is influenced by the research and reports that industry or securities analysts publish about us or our business as well as the stock indices that our common stock is included in. If any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, or if the S&P 600 removes us from its index, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
| 32 |
We may raise capital through the issuance of our common stock, convertible debt or equity linked securities, which could result in dilution to our stockholders or negatively impact the price of our common stock.
In August 2020 we issued 2,090,909 shares of our common stock in a follow-on public offering. We may choose to raise additional capital due to market conditions or strategic considerations. To the extent that additional capital is raised through the sale of equity, convertible debt or other equity linked securities, the issuance of these securities could result in dilution to our stockholders or result in downward pressure on the price of our common stock.
ITEM 1B – UNRESOLVED STAFF COMMENTS
None.
ITEM 2 – PROPERTIES
Our corporate headquarters is in Lancaster, California, where we lease 9,255 square feet of office space. The term of the lease extends to January 31, 2026 and the base rent is approximately $17 thousand per month. The lease agreement gives the Company the right, upon 180 days’ prior notice, to opt out of all or part of the last four years of the term, with no penalty.
We lease 12,623 square feet of office space in Buffalo, New York. The initial five-year lease term expired in October 2018 and was renewed for a three-year option, extending it to November 2021, at a base rent of approximately $16 thousand per month. On August 3, 2021, a new lease agreement for a different property was signed for a five-year term at a base rent of approximately $7 thousand per month with an annual 2% increase, and with two, five-year renewal options. Due to ongoing construction, the Company has not yet moved into the new property but anticipates doing so and commencing the lease term no later than November 2021.
We lease approximately 2,700 square feet of space in Research Triangle Park, North Carolina. The initial three-year term was due to expire in October 2020. An amendment to the initial lease became effective April 1, 2020, which added 686 square feet and extended the term of the lease to September 30, 2023. The new base rent is approximately $8 thousand per month with an annual 3% increase.
We lease approximately 2,300 square feet of office space in Paris, France. As of April 1, 2020, the lease agreement had minimum payments equaling approximately $288 thousand. The lease is for a 9-year term, with an option to terminate every 3 years, and expires in November of 2024. The base rent is $16 thousand per quarter (approximately $5.3 thousand per month) and can be adjusted each December based on a consumer price index.
Rent expense, including common area maintenance fees for the years ended August 31, 2021, 2020, and 2019 was $655 thousand, $644 thousand, and $584 thousand, respectively.
The Company believes its existing facilities and equipment are in good operating condition and are suitable for the conduct of its business.
| 33 |
ITEM 3 – LEGAL PROCEEDINGS
We may become subject to litigation, claims, investigations, and audits arising from time to time in the ordinary course of our business. At this time, however, we are not a party to any legal proceedings and are not aware of pending legal proceedings.
ITEM 4 – MINE SAFETY DISCLOSURES.
Not applicable.
| 34 |
PART II
ITEM 5 – MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
The Company’s common stock, par value $0.001 per share, has traded on the Nasdaq Global Select Market under the symbol “SLP” since May 13, 2021, prior to which it traded on the Nasdaq Capital Market under the same symbol.
Holders
As of October 25, 2021, there were 43 shareholders of record. A substantially greater number of holders of our common stock are “street name” or beneficial holders, whose shares are held by banks, brokers and other financial institutions.
Dividends
The following dividends were declared by our Board of Directors during the fourth quarter of fiscal year 2021:
(in thousands, except dividend per share amounts)
| Fiscal Year | Record Date | Distribution Date | # of Shares Outstanding on Record Date | Dividend per Share | Total Amount | |||||||||
| 2021 | 7/26/2021 | 8/02/2021 | 20,139 | $ | 0.06 | $ | 1,208 | |||||||
Although we expect to pay quarterly dividends of $0.06 per share of common stock each quarter, the dividend is subject to declaration by our Board of Directors. There can be no assurances that our Board of Directors will continue the dividend distributions for any specified number of quarters. Refer to Note 8 – Shareholders’ Equity of the Notes to Financial Statements (Part II, Item 8 of this Annual Report on Form 10-K) for further details regarding dividends.
| 35 |
Shareholder Return Performance Graph
The following graph compares the cumulative total stockholder return on our common stock of a $100 investment from August 31, 2017 through August 31, 2021, assuming reinvestment of dividends, with a similar investment in the Russell 3000 index (the “Russell 3000”) and with the companies listed in the Nasdaq Composite - Total Returns (“IXIC”), and the S&P600 Health Care Equipment & Services Industry Group Index (SP600-3510). The historical information set forth below is not necessarily indicative of future performance. This performance graph shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or incorporated by reference into any of our filings under the Securities Act of 1933, as amended, of the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
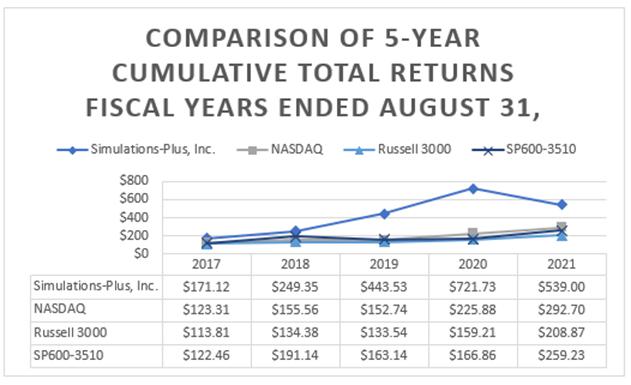
Recent Sales of Unregistered Securities; Use of Proceeds from Registered Securities
On June 1, 2021, we issued an aggregate of 11,540 unregistered shares of our common stock to the former owners of Lixoft pursuant to that Share Purchase and Contribution Agreement, dated March 31, 2020, entered into between the Company and such former owners. The shares had an aggregate value of $666 thousand and were issued as an earnout payment in connection with the satisfaction of certain year-over-year performance thresholds set forth in the Share Purchase and Contribution Agreement.
The shares of common stock were issued in a transaction not involving a public offering in reliance upon an exemption from registration provided by Section 4(a)(2) of the Securities Act, and/or Regulation S promulgated thereunder.
During the fiscal year ended August 31, 2021, there were no other unregistered sales of our securities that were not reported in a Current Report on Form 8-K or our Quarterly Reports on Form 10-Q.
Repurchases
There is currently no share repurchase program pending, and the Company has made no repurchases of its securities since fiscal year 2011; however, the Board of Directors may decide to institute such a program in the future.
| 36 |
ITEM 6 – [RESERVED]
ITEM 7 – MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis is intended to assist the reader in understanding our results of operations and financial condition. Management’s Discussion and Analysis is provided as a supplement to, and should be read in conjunction with, our audited consolidated financial statements beginning on page F-1 of this Annual Report on Form 10-K. This Annual Report on Form 10-K includes certain statements that may be deemed to be “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended. All statements, other than statements of historical fact, included in this Annual Report on Form 10-K that address activities, events or developments that we expect, project, believe, or anticipate will or may occur in the future, including matters having to do with expected and future revenue, our ability to fund our operations and repay debt, business strategies, expansion and growth of operations and other such matters, are forward-looking statements. These statements are based on certain assumptions and analyses made by our management in light of its experience and its perception of historical trends, current conditions, expected future developments, and other factors it believes are appropriate in the circumstances. These statements are subject to a number of assumptions, risks and uncertainties, including general economic and business conditions, the business opportunities (or lack thereof) that may be presented to and pursued by us, our performance on our current contracts and our success in obtaining new contracts, our ability to attract and retain qualified employees, and other factors, many of which are beyond our control. You are cautioned that these forward-looking statements are not guarantees of future performance and those actual results or developments may differ materially from those projected in such statements.
Management Overview
Fiscal Year 2021 Financial Highlights:
| · | Consolidated revenues increased by $4.9 million, or 11.7%, to $46.5 million for the year ended August 31, 2021, compared to $41.6 million for the year ended August 31, 2020. | |
| · | Consolidated gross profit increased by approximately $5.0 million or 15.9%, to $35.9 million for the year ended August 31, 2021, compared to $30.9 million for the year ended August 31, 2020. | |
| · | Income from operations decreased by $352 thousand, or 3.0%, to approximately $11.3 million for the year ended August 31, 2021, from $11.6 million for the year ended August 31, 2020. Fiscal year 2020 includes a one-time acquisition cost of $1.4 million related to Lixoft. | |
| · | Net income increased by $450 thousand or 4.8% to $9.8 million for the year ended August 31, 2021, compared to $9.3 million for the year ended August 31, 2020. | |
| · | Diluted earnings per share decreased by $0.03 or 6.0% to $0.47 for the year ended August 31, 2021, compared to $0.50 for the year ended August 31, 2020. |
Strategy Going Forward:
| · | Continue to pursue funded and unfunded collaborations in support of improving our products and services | |
| · | Continue our aggressive marketing and sales campaign | |
| · | Continue to expand our use of social media and advertising | |
| · | Continue to expand our sales staff, both in-house and in the field | |
| · | Continue to recruit scientific and other resources to support our product and scientific consulting services | |
| · | Seek accretive acquisitions that complement our existing offerings and expand our markets |
| 37 |
Fiscal year 2021 was yet another record year for the Company. We saw good growth in the midst of the fiscal year that had to bear the brunt of the COVID-19 global pandemic. We believe the continued growth of our pharmaceutical software and services business is the result of steadily increasing adoption and awareness of the value of simulation and modeling software tools across the pharmaceutical industry, the continuing push by regulatory agencies for increased use of modeling and simulation, and the expertise we offer as consultants to assist companies involved in the research and development of new medicines. We continue to be a leader in the fast- growing $2 billion bio-simulation industry.
Results of Operations
A discussion regarding our financial condition and results of operations for fiscal 2019 compared to fiscal 2018 can be found under Item 7 in our Annual Report on Form 10-K for the fiscal year ended August 31, 2019, filed with the SEC on November 13, 2019, which is available free of charge on the SEC’s website at www.sec.gov and our corporate website at https://www.simulations-plus.com/investorscorporate-profile/sec-filings/.
Comparison of fiscal year 2021 and fiscal year 2020
| (in thousands) | Year Ended August 31, | |||||||||||||||
| 2021 | 2020 | $ Change | % Change | |||||||||||||
| Revenues | $ | 46,466 | $ | 41,589 | $ | 4,877 | 12 % | |||||||||
| Cost of revenues | 10,600 | 10,649 | (49 | ) | (1)% | |||||||||||
| Gross profit | 35,866 | 30,940 | 4,926 | 16 % | ||||||||||||
| Research and development | 4,047 | 2,975 | 1,072 | 36 % | ||||||||||||
| Selling, general and administrative | 20,566 | 16,360 | 4,206 | 26 % | ||||||||||||
| Total operating expenses | 24,613 | 19,335 | 5,278 | 27 % | ||||||||||||
| Income from operations | 11,253 | 11,605 | (352 | ) | (3)% | |||||||||||
| Other income (expense), net | (168 | ) | (218 | ) | 50 | (23)% | ||||||||||
| Income before income taxes | 11,085 | 11,387 | (302 | ) | (3)% | |||||||||||
| Provision for income taxes | (1,303 | ) | (2,055 | ) | 752 | (37)% | ||||||||||
| Net income | $ | 9,782 | $ | 9,332 | $ | 450 | 5 % | |||||||||
Revenues
Revenues increased by approximately $4.9 million or 12% to $46.5 million for the year ended August 31, 2021, compared to approximately $41.6 million for the year ended August 31, 2020. This increase is primarily due to a $6.1 million or 28% increase in software-related revenue, offset by a $1.2 million or 6% decrease in consulting services and analytical study revenue when comparing the years ended August 31, 2021, and 2020.
Cost of revenues
Cost of revenues remained relatively consistent with a slight decrease of $49 thousand or approximately 1% for the year ended August 31, 2021, compared to the year ended August 31, 2020. The decrease is primarily due to lower contract research organization fees of $204 thousand, lower tech-support costs of $135 thousand, lower labor-related costs of $100 thousand, and lower training and travel costs of $97 thousand, partially offset by higher amortization of software development costs of $455 thousand related to the purchase of Lixoft.
A significant portion of cost of revenues for pharmaceutical software products is the systematic amortization of capitalized software development costs, which is a fixed cost rather than a variable cost related to revenues. The amortization cost of $2.8 million for the year ended August 31, 2021, increased by approximately $455 thousand compared to fiscal year 2020.
Cost of revenues as a percentage of revenue was 22.8% for the year ended August 31, 2021, compared to 25.6% for the year ended August 31, 2020, resulting in a decrease of 2.8%.
| 38 |
Gross profit
Gross profit increased by approximately $5.0 million or 16% to $35.9 million for the year ended August 31, 2021, compared to approximately $30.9 million for the year ended August 31, 2020. The increase is due to an increase in gross profit for the software business of $5.7 million or 31%, partially offset by a decrease in gross profit for the consulting services business of $0.7 million or 7% over the same periods.
Overall gross margin percentage increased by 2.8% to 77.2% for the year ended August 31, 2021, from 74.4% for the year ended August 31, 2020.
Research and development
We incurred approximately $6.9 million of research and development costs during the year ended August 31, 2021. Of this amount, $2.9 million was capitalized and $4.0 million was expensed. We incurred approximately $5.3 million of research and development costs during year ended August 31, 2020. Of this amount, $2.3 million was capitalized and $3.0 million was expensed. The year-over-year increase of $1.6 million, or 30%, in research and development expenditures was primarily due to increased costs in the Simulations Plus, DILIsym and Lixoft divisions.
Selling, general and administrative expenses
Selling, general, and administrative (“SG&A”) expenses increased by $4.2 million, or 26% to $20.6 million for the year ended August 31, 2021, compared to $16.4 million for the year ended August 31, 2020, primarily due to the following:
| · | An increase in salaries and wages of $3.3 million due to higher corporate salaries, bonuses, stock-based compensation, and 401K costs, as well as an increase in headcount. | |
| · | An increase in payroll tax expense of $707 thousand, resulting from higher salary and wage related costs. |
As a percent of revenues, SG&A expense was 44% for the year ended August 31, 2021, compared to 39% for the year ended August 31, 2020.
Other income/expense
Total other expense was $168 thousand for the year ended August 31, 2021, compared to total other expense of $218 thousand for the year ended August 31, 2020. The variance of $50 thousand is primarily due to an increase in currency exchange gain $184 thousand and an increase in interest income of $171 thousand, offset by an increase in the change in the value of contingent consideration of $283 thousand.
Provision for income taxes
The provision for income taxes was $1.3 million for the year ended August 31, 2021, compared to $2.1 million for the year ended August 31, 2020. Our effective tax rate decreased by 6.2% to 11.8% for the year ended August 31, 2021, from 18.0% for the year ended August 31, 2020.
The effective rate differs from anticipated combined statutory rates of approximately 24.5% due to R&D credits, foreign-tax related items (tax credits and foreign-deemed intangible income deductions), and the tax effect of stock-compensation related items for stock compensation and disqualifying dispositions. During the years ended August 31, 2021, and 2020, as a result of an increase in stock prices, a number of employees exercised and sold incentive stock options granted to them under their corporate incentive plans, creating corporate tax deductions that lowered the effective tax rate.
| 39 |
Comparison of fiscal year 2020 and fiscal year 2019
| (in thousands) | Year Ended August 31, | |||||||||||||||
| 2020 | 2019 | $ Change | % Change | |||||||||||||
| Revenues | $ | 41,589 | $ | 33,970 | $ | 7,619 | 22% | |||||||||
| Cost of revenues | 10,649 | 9,026 | 1,623 | 18% | ||||||||||||
| Gross profit | 30,940 | 24,944 | 5,996 | 24% | ||||||||||||
| Research and development | 2,975 | 2,500 | 475 | 19% | ||||||||||||
| Selling, general and administrative | 16,360 | 11,796 | 4,564 | 39% | ||||||||||||
| Total operating expenses | 19,335 | 14,296 | 5,039 | 35% | ||||||||||||
| Income from operations | 11,605 | 10,648 | 957 | 9% | ||||||||||||
| Other income (expense), net | (218 | ) | (92 | ) | (126 | ) | 137% | |||||||||
| Income before income taxes | 11,387 | 10,556 | 831 | 8% | ||||||||||||
| Provision for income taxes | (2,055 | ) | (1,973 | ) | (82 | ) | 4% | |||||||||
| Net income | $ | 9,332 | $ | 8,583 | $ | 749 | 9% | |||||||||
Revenues
Revenues increased by approximately $7.6 million or 22% to $41.6 million for the year ended August 31, 2020 compared to $34.0 million for the year ended August 31, 2019. This increase is primarily due to a $4.5 million or 29.1% increase in consulting services revenue. Software-related revenue increased $3.1 million or 16.8% when comparing the fiscal years ended August 31, 2020 and 2019.
Cost of Revenues
Cost of revenues increased by approximately $1.6 million or 18% to $10.6 million for the year ended August 31, 2020 compared to $9.0 million for the year ended August 31, 2019. The higher cost is primarily due to an increase in consulting-related labor costs of $1.6 million.
A significant portion of cost of revenues for pharmaceutical software products is the systematic amortization of capitalized software development costs, which is a fixed cost rather than a variable cost related to revenues. The amortization cost for fiscal year 2020 was $2.4 million, an increase of $103 thousand compared to fiscal year 2019.
Cost of revenues as a percentage of revenue decreased to 25.6% in fiscal year 2020 from 26.6% in fiscal year 2019.
Gross profit
Gross profit increased $6.0 million or 24% to $30.9 million for the year ended August 31, 2020, compared to $24.9 million for the year ended August 31, 2019. The increase is due to an increase in gross profit for the software business of $3.2 million or 20%, as well as an increase in gross profit for the consulting services business of $2.8 million or 30% over the same periods.
Overall gross margin percentage increased by 1% to 74% for the year ended August 31, 2020, compared to the year ended August 31, 2019.
Research and Development
We incurred approximately $5.3 million of research and development costs during year ended August 31, 2020. Of this amount, approximately $2.3 million was capitalized and $3.0 million was expensed. We incurred approximately $4.3 million of research and development costs during year ended August 31, 2019. Of this amount, $1.8 million was capitalized and $2.5 million was expensed. The increase of approximately $1.0 million in total research and development expenditures in fiscal year 2020 compared to fiscal year 2019 was primarily due to increased costs in the Simulations Plus and DILIsym divisions.
| 40 |
Selling, General and Administrative Expenses
SG&A expenses increased by $4.6 million, or 39% to $16.4 million for the year ended August 31, 2020 compared to $11.8 million for the year ended August 31, 2019. The increase was primarily due to a $1.5 million increase in general and administrative salaries; $1.4 million in legal, accounting and consulting fees associated with the Lixoft acquisition; an increase in payroll tax expense of $478 thousand; an increase in director compensation of $394 thousand due to additional paid directors and increases in compensation; an increase in insurance costs of $259 thousand due to higher headcount and a $226 thousand increase in commission costs related to increased revenues domestically and in Asia.
As a percent of revenues, selling, general and administrative expenses was 39.3% for fiscal year 2020, compared to 34.7% for fiscal year 2019.
Other income/expense
Total other expense was $218 thousand for the year ended August 31, 2020 compared to $92 thousand for the year ended August 31, 2019. The variance of $126 thousand is primarily due to a change in the valuation of contingent consideration.
Provision for Income Taxes
The provision for income taxes was approximately $2.1 million for the year ended August 31, 2020, compared to $2.0 million for the year ended August 31, 2019. Our effective tax rate decreased slightly to 18.0% from 18.7 % for the same periods.
The effective rate differs from anticipated combined statutory rates of approximately 25.7% due to R&D credits, foreign-tax related items (tax credits and foreign-deemed intangible income deductions), and the tax effect of stock-compensation related items for stock compensation and disqualifying dispositions. In the last part of fiscal year 2020, as occurred also in fiscal year 2019, because of an increase in stock prices, a number of employees exercised and sold incentive stock options granted to them under their corporate incentive plans, creating corporate tax deductions that lowered the effective tax rate.
Segment Results of Operations
Comparison of fiscal year 2021 and fiscal year 2020
Revenues
| (in thousands) Year Ended August 31, | ||||||||||||||||
| 2021 | 2020 | Change ($) | Change (%) | |||||||||||||
| Simulations Plus | $ | 25,142 | $ | 21,961 | $ | 3,181 | 14 % | |||||||||
| Cognigen | 10,546 | 11,105 | (559 | ) | (5)% | |||||||||||
| DILIsym | 6,115 | 6,948 | (833 | ) | (12)% | |||||||||||
| Lixoft* | 4,663 | 1,575 | 3,088 | 196 % | ||||||||||||
| Total | $ | 46,466 | $ | 41,589 | $ | 4,877 | 12 % | |||||||||
*As Lixoft was acquired on April 1, 2020, five months of activity is reflected for fiscal year 2020.
| 41 |
Cost of Revenues
| (in thousands) Year Ended August 31, | ||||||||||||||||
| 2021 | 2020 | Change ($) | Change (%) | |||||||||||||
| Simulations Plus | $ | 3,001 | $ | 2,921 | $ | 80 | 3 % | |||||||||
| Cognigen | 4,825 | 5,190 | (365 | ) | (7)% | |||||||||||
| DILIsym | 2,036 | 2,271 | (235 | ) | (10)% | |||||||||||
| Lixoft* | 738 | 267 | 471 | 176 % | ||||||||||||
| Total | $ | 10,600 | $ | 10,649 | $ | (49 | ) | (1)% | ||||||||
* As Lixoft was acquired on April 1, 2020, five months of activity is reflected for fiscal year 2020.
Gross Profit
| (in thousands) Year Ended August 31, | ||||||||||||||||
| 2021 | 2020 | Change ($) | Change (%) | |||||||||||||
| Simulations Plus | $ | 22,141 | $ | 19,040 | $ | 3,101 | 16 % | |||||||||
| Cognigen | 5,721 | 5,915 | (194 | ) | (3)% | |||||||||||
| DILIsym | 4,079 | 4,677 | (598 | ) | (13)% | |||||||||||
| Lixoft* | 3,925 | 1,308 | 2,617 | 200 % | ||||||||||||
| Total | $ | 35,866 | $ | 30,940 | $ | 4,926 | 16 % | |||||||||
* As Lixoft was acquired on April 1, 2020, five months of activity is reflected for fiscal year 2020.
Simulations Plus
For the year ended August 31, 2021, the revenues increase of $3.2 million or 14% compared to the year ended August 31, 2020, was primarily due to higher revenues from GastroPlus of $2.2 million and an increase in revenues from ADMET Software of $831 thousand. Cost of revenue increased marginally during the same periods, and gross profit increased by $3.1 million or 16%, primarily due to the increase in revenue.
Cognigen
For the year ended August 31, 2021, the revenue decrease of $559 thousand or 5% compared to the year ended August 31, 2020, was primarily due to a decrease in grant revenue of $672 thousand, partially offset by an increase in training revenue of $90 thousand. Cost of revenue decreased $365 thousand or 7%, primarily due to lower salary cost related to employees working on service contracts of $726 thousand, partially offset by an increase in consulting related costs of $346 thousand. Gross profit decreased by approximately $194 thousand or 3% for the same periods.
| 42 |
DILIsym
For the year ended August 31, 2021, the revenue decrease of $833 thousand or 12% compared to the year ended August 31, 2020, was primarily due to lower revenue from consulting services of $869 thousand. Cost of revenue decreased by $235 thousand or 10% during the same periods, primarily due to lower contract research organization fees of $204 thousand. Gross profit decreased by $598 thousand or 13%.
Lixoft
For the year ended August 31, 2021, the revenue increase of $3.1 million compared to the year ended August 31, 2020 was primarily due to an increase in revenues from MonolixSuite of $2.9 million; this increase was primarily the result of the purchase of Lixoft on April 1, 2020. Software sales of the MonolixSuite generated 97% of total revenue and consulting services generated 3% of total revenue. Cost of revenue increased by $471 thousand, and gross profit increased by $2.6 million also due to the purchase of Lixoft on April 1, 2020.
Comparison of fiscal year 2020 and fiscal year 2019
Revenues
| (in thousands) Year Ended August 31, | ||||||||||||||||
| 2020 | 2019 | Change ($) | Change (%) | |||||||||||||
| Simulations Plus | $ | 21,961 | $ | 19,584 | $ | 2,377 | 12 % | |||||||||
| Cognigen | 11,105 | 9,321 | 1,784 | 19 % | ||||||||||||
| DILIsym | 6,948 | 5,065 | 1,883 | 37 % | ||||||||||||
| Lixoft* | 1,575 | – | 1,575 | – | ||||||||||||
| Total | $ | 41,589 | $ | 33,970 | $ | 7,619 | 22 % | |||||||||
*As Lixoft was acquired on April 1, 2020, five months of activity is reflected for fiscal year 2020.
Cost of Revenues
| (in thousands) Year Ended August 31, | ||||||||||||||||
| 2020 | 2019 | Change ($) | Change (%) | |||||||||||||
| Simulations Plus | $ | 2,921 | $ | 3,276 | $ | (355 | ) | (11)% | ||||||||
| Cognigen | 5,190 | 4,366 | 824 | 19 % | ||||||||||||
| DILIsym | 2,271 | 1,384 | 887 | 64 % | ||||||||||||
| Lixoft* | 267 | – | 267 | – | ||||||||||||
| Total | $ | 10,649 | $ | 9,026 | $ | 1,623 | 18 % | |||||||||
*As Lixoft was acquired on April 1, 2020, five months of activity is reflected for fiscal year 2020.
| 43 |
Gross Profit
| (in thousands) Year Ended August 31, | ||||||||||||||||
| 2020 | 2019 | Change ($) | Change (%) | |||||||||||||
| Simulations Plus | $ | 19,040 | $ | 16,308 | $ | 2,732 | 17 % | |||||||||
| Cognigen | 5,915 | 4,955 | 960 | 19 % | ||||||||||||
| DILIsym | 4,677 | 3,681 | 996 | 27 % | ||||||||||||
| Lixoft* | 1,308 | – | 1,308 | – | ||||||||||||
| Total | $ | 30,940 | $ | 24,944 | $ | 5,996 | 24 % | |||||||||
*As Lixoft was acquired on April 1, 2020, five months of activity is reflected for fiscal year 2020.
Simulations Plus
For the year ended August 31, 2020, the revenue increase of $2.4 million or 12% compared to the year ended August 31, 2019 was primarily due to increases in revenue from GastroPlus of $1.3 million, from ADMET Software of $630 thousand and from services revenue totaling $525 thousand. The cost of revenue decrease of $355 thousand or 11% during the same periods was primarily due to lower royalty expense of $222 thousand resulting from the renegotiation of the agreement with Dassault Systemes Americas Corp. in June 2019 and a decrease in amortization expense of capitalized software of $98 thousand. Gross profit increased by $2.7 million or 17%, primarily due to the increase in revenue.
Cognigen
For the year ended August 31, 2020, the revenue increase of $1.8 million or 19% compared to the year ended August 31, 2019 was primarily due to an increase in grant revenue. Cost of revenue increased by $824 thousand or 19%, primarily due to an increase in salary contracts of $367 thousand and higher international subcontractor costs of $335 thousand. Gross profit increased by approximately $960 thousand or 19% for the same periods.
DILIsym
For the year ended August 31, 2020, the revenue increase of $1.9 million or 37% compared to the year ended August 31, 2019, was primarily due to higher revenue from consulting services of $2.1 million, partially offset by lower licensing revenue of $114 thousand. Cost of revenue increased by $887 thousand or 64% during the same periods, primarily due to higher salary cost of $277 thousand, higher contract research organization fees of $274 thousand, and higher bonus accrual of $160 thousand. Gross profit increased by approximately $1.0 million or 27% for the same periods.
Lixoft
For the year ended August 31, 2020, the revenue increase of $1.6 million compared to the August 31, 2019 was due to the purchase of Lixoft on April 1, 2020. Software sales of the MonolixSuite generated 98% of total revenue and 2% was generated from consulting services. Cost of revenue increased $267 thousand, and gross profit increased $1.3 million primarily due to the purchase of Lixoft on April 1, 2020.
| 44 |
LIQUIDITY AND CAPITAL RESOURCES
As of August 31, 2021, the Company had $37.0 million in cash and cash equivalents and $86.6 million in short-term investments. Our principal sources of capital have been cash flows from our operations and a public offering in 2020. We have achieved continuous positive operating cash flow over the last twelve fiscal years.
In August 2020, the Company closed an underwritten public offering of 2,090,909 shares of its common stock to the public at $55.00 per share, which included the full exercise of the underwriters’ option to purchase 272,727 additional shares of common stock. The aggregate gross proceeds to the Company from this offering were approximately $115.0 million, before deducting underwriting discounts and commissions; net proceeds were approximately $107.7 million. The Company has used, and intends to continue to use the net proceeds from the offering for strategic mergers and acquisitions (although the Company has no present commitments or agreements to enter into any such mergers or acquisitions), working capital requirements, and other general corporate purposes, including investing in enhanced information and accounting systems, and personnel in support of corporate growth. The offering was made pursuant to our automatic shelf registration statement on Form S-3 filed with the SEC on July 9, 2020.
On March 31, 2020, the Company entered into a Stock Purchase and Contribution Agreement (the “Lixoft Agreement”) with Lixoft, a French société par actions simplifiée (“Lixoft”). On April 1, 2020, the Company consummated the acquisition of all outstanding equity interests of Lixoft pursuant to the terms of the Lixoft Agreement, with Lixoft becoming a wholly owned subsidiary of the Company. Under the terms of the Agreement, the Company will pay the former shareholders of Lixoft total consideration of up to $16.5 million, consisting of two-thirds cash and one-third newly issued, unregistered shares of the Company’s common stock. In addition, the Company paid approximately $3.5 million of excess working capital based on the March 31, 2020 financial statements of Lixoft. As part of the total consideration, the agreement calls for earnout payments up to an additional $5.5 million, two-thirds cash and one-third newly issued, unregistered shares of the Company’s common stock based on a revenue growth formula each year for the two years subsequent to April 1, 2020. The former shareholders earned $2.0 million in the first year and can earn up to $3.5 million in year two. See Note 14 for a further description of the Lixoft Agreement.
We believe that our existing capital and anticipated funds from operations will be sufficient to meet our anticipated cash needs for working capital and capital expenditures for the foreseeable future. Thereafter, if cash generated from operations is insufficient to satisfy our capital requirements, we may draw from our revolving line of credit with the bank, or we may have to sell additional equity or debt securities or obtain expanded credit facilities. In the event such financing is needed in the future, there can be no assurance that such financing will be available to us, or, if available, that it will be in amounts and on terms acceptable to us. If cash flows from operations became insufficient to continue operations at the current level, and if no additional financing was obtained, then management would restructure the Company in a way to preserve its pharmaceutical business while maintaining expenses within operating cash flows.
We continue to seek opportunities for strategic acquisitions. If one or more such acquisitions is identified, a substantial portion of our cash reserves may be required to complete it; however, we intend to maintain sufficient cash reserves after any acquisition to provide reasonable assurance that outside financing will not be necessary to continue operations. If we identify an attractive acquisition that would require more cash to complete than we are willing or able to use from our cash reserves, we will consider financing options to complete the acquisition, including obtaining loans and issuing additional securities.
We are not aware of any trends or demands, commitments, events or uncertainties that are reasonably likely to result in a decrease in liquidity of our assets. The trend over the last ten years has been increasing cash deposits from our operating cash flows, and we expect that trend to continue for the foreseeable future.
| 45 |
Cash Flows
Operating Activities
Net cash provided by operating activities was $19.2 million for the year ended August 31, 2021. Our operating cash flows resulted primarily from our net income of $9.8 million, which was generated by cash received from our customers, offset by cash payments we made to third parties for their services and employee compensation. In addition, net cash inflow from changes in balances of operating assets and liabilities was $1.0 million, and non-cash charges were $8.4 million. The change in operating assets and liabilities was primarily the result of an increase in accrued payroll and other expenses, partially offset by an increase in accounts receivable.
Net cash provided by operating activities was $10.9 million for the year ended August 31, 2020. Our operating cash flows resulted primarily from our net income of $9.3 million, which was generated by cash received from our customers, offset by cash payments we made to third parties for their services and employee compensation. In addition, net cash outflow from changes in balances of operating assets and liabilities was $2.8 million, offset by non-cash charges of $4.4 million. The change in operating assets and liabilities was primarily the result of an increase in accounts receivable and a decrease in billings in excess of revenues.
Investing Activities
Net cash used in investing activities during the year ended August 31, 2021, of $26.7 thousand was primarily due to the purchase of short-term investments of $122.4 million and computer software development costs of $2.9 million, partially offset by proceeds from the sale of short-term investments totaling $100.2 million.
Cash used for investing activities during the year ended August 31, 2020 of $75.5 million was primarily due to the purchase of $67.2 million of short-term investments and costs associated with the acquisition of a subsidiary totaling $9.5 million.
Financing Activities
For the year ended August 31, 2021, net cash used in financing activities of $4.7 million, was primarily due to dividend payments totaling $4.8 million and a $1.3 million earnout payment to the former shareholders of Lixoft, partially offset by proceeds from the exercise of stock options totaling $1.5 million.
Net cash provided by financing activities during the year ended August 31, 2020 of $102.4 million was primarily due to the net proceeds from a public offering of $107.7 million, partially offset by dividend payments totaling $4.3 million for the period.
DIVIDENDS
Refer to Note 8 – Shareholders’ Equity of the Notes to Financial Statements (Part II, Item 8 of this Form 10-K) for details regarding dividends.
KNOWN TRENDS OR UNCERTAINTIES
Although we have not seen any significant reduction in total revenues to date, we did see a reduction in PKPD services during the year ended August 31, 2021, primarily resulting from project disruptions due to customer delays, holds, and drug development program cancellations. We have also seen consolidation in the pharmaceutical industry during economic downturns, although these consolidations have not had a negative effect on our total revenues to that industry. Should customer delays, holds, program cancellations, or consolidations and downsizing in the industry continue to occur, those events could adversely impact our revenues and earnings going forward.
As discussed in the Risk Factor section of this Annual Report on Form 10-K, the world has been affected due to the COVID-19 pandemic. Although there has not been a substantial impact on revenues to date, until the pandemic has passed, there remains uncertainty as to the effect on our business in both the short and long-term.
| 46 |
We believe that the need for improved productivity in the research and development activities directed toward developing new medicines will continue to result in increasing adoption of simulation and modeling tools such as those we produce. New product developments in the pharmaceutical business segments could result in increased revenues and earnings if they are accepted by our markets; however, there can be no assurances that new products will result in significant improvements to revenues or earnings. For competitive reasons, we do not disclose all of our new product development activities.
Our continued quest for acquisitions could result in a significant change to revenues and earnings if one or more such acquisitions are completed.
The potential for growth in new markets (e.g., healthcare) is uncertain. We will continue to explore these opportunities until such time as we either generate revenues or determine that resources would be more efficiently used elsewhere.
OFF-BALANCE SHEET ARRANGEMENTS
As of August 31, 2021, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured-finance or special-purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As such, we are not materially exposed to any financing, liquidity, market, or credit risk that could arise if we had engaged in such relationships.
We do not have relationships or transactions with persons or entities that derive benefits from their non-independent relationship with us or our related parties.
CONTRACTUAL OBLIGATIONS
The following table provides aggregate information regarding our contractual obligations as of August 31, 2021:
| (in thousands) | Payments due by period | |||||||||||||||||||
| Contractual obligations: | Total | 1 year | 2–3 years | 4–5 years | More than 5 years | |||||||||||||||
| Contracts payable(1) | $ | 4,550 | $ | 4,550 | $ | – | $ | – | $ | – | ||||||||||
| Operating lease obligations(2) | 1,366 | 422 | 650 | 294 | – | |||||||||||||||
| Total | $ | 5,916 | $ | 4,972 | $ | 650 | $ | 294 | $ | – | ||||||||||
| (1) | Contracts payable are related to our Stock Purchase and Contribution Agreement that the Company entered into with Lixoft on March 31, 2020. Under the terms of the agreement, we agreed to pay the former shareholders of Lixoft earnout payments up to an $5.5 million, two-thirds cash and one-third newly issued, unregistered shares of the Company’s common stock based on a revenue growth formula each year for the two years subsequent to April 1, 2020. For further details regarding our contracts payable, refer to Note 6 and Note 14 to the “Notes to Consolidated Financial Statements” in Part II, Item 8 of this of this Annual Report on Form 10-K. |
| (2) | Operating lease obligations relate to our office space and facilities. The lease terms expire in various years through 2026 and are generally renewable at our option. For more information on our operating lease, refer to Note 7 to the “Notes to Consolidated Financial Statements” in Part II, Item 8 of this of this Annual Report on Form 10-K. |
We believe that our current cash and cash equivalents and cash generated from operations will be sufficient to meet our working capital, capital expenditures and contractual obligation requirements.
| 47 |
RECENTLY ISSUED OR NEWLY ADOPTED ACCOUNTING STANDARDS
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes existing guidance on accounting for leases in "Leases (Topic 840)" and generally requires all leases to be recognized in the consolidated balance sheet. ASU 2016-02 is effective for annual and interim reporting periods beginning after December 15, 2018. The Company adopted this ASU on September 1, 2019.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which is intended to simplify various areas related to the accounting for income taxes and improve consistent application of Topic 740. The guidance eliminates certain exceptions related to the approach for intra-period tax allocation, the methodology for calculating income taxes in an interim period, and the recognition of deferred tax liabilities for outside basis differences related to changes in ownership of equity-method investments and foreign subsidiaries. The guidance also simplifies aspects of accounting for franchise taxes and the accounting for the enacted changes in tax laws or rates, as well as the accounting for the step-up in the tax basis of goodwill. ASU 2019-12 is effective for us beginning in fiscal 2022. The adoption of the new standard is not expected to have a material impact on the Company’s consolidated financial statements.
In March 2020, the FASB issued Accounting Standards Update (“ASU”) 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting (“ASU 2020-04”). The amendments in ASU 2020-04 provide temporary optional expedients and exceptions for applying GAAP to contract modifications, hedging relationships and other transactions to ease the potential accounting and financial reporting burden associated with transitioning away from reference rates that are expected to be discontinued, including the London Interbank Offered Rate (“LIBOR”). This ASU is effective as of March 12, 2020, through December 31, 2022. The adoption of the new standard has not had and is not expected to have a material impact on our financial statements or related disclosures.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Estimates
Our financial statements and accompanying notes are prepared in accordance with GAAP. Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. These estimates and assumptions are affected by management’s application of accounting policies. Actual results could differ from those estimates. Significant accounting policies for us include revenue recognition, accounting for capitalized software development costs, valuation of stock options, and accounting for income taxes.
Revenue Recognition
We generate revenue primarily from the sale of software licenses and providing consulting services to the pharmaceutical industry for drug development.
The Company determines revenue recognition through the following steps:
| i. | Identification of the contract, or contracts, with a customer |
| ii. | Identification of the performance obligations in the contract |
| iii. | Determination of the transaction price |
| iv. | Allocation of the transaction price to the performance obligations in the contract |
| v. | Recognition of revenue when, or as, the Company satisfies a performance obligation |
The Company accounts for a contract when it has approval and commitment from both parties, the rights of the parties are identified, payment terms are identified, the contract has commercial substance and collectability of consideration is probable. Contracts generally have fixed pricing terms and are not subject to variable pricing. The Company considers the nature and significance of each specific performance obligation under a contract when allocating the proceeds under each contract. Accounting for contracts includes significant judgement in the estimation of estimated hours/cost to be incurred on consulting contracts, and the di minimis nature of the post-sales costs associated with software sales.
| 48 |
Capitalized Computer Software Development Costs
Software development costs are capitalized in accordance with FASB ASC 985-20, “Costs of Software to Be Sold Leased, or Marketed”. Capitalization of software development costs begins upon the establishment of technological feasibility and is discontinued when the product is available for sale.
The establishment of technological feasibility and the ongoing assessment for recoverability of capitalized computer software development costs require considerable judgment by management with respect to certain external factors including, but not limited to, technological feasibility, anticipated future gross revenues, estimated economic life, and changes in software and hardware technologies. Capitalized software development costs are comprised primarily of salaries and direct payroll-related costs and the purchase of existing software to be used in the Company’s software products. Total capitalized computer software development costs were $2.9 million, $2.4 million and $1.8 million for the fiscal years ending August 31, 2021, 2020 and 2019, respectively.
Amortization of capitalized computer software development costs is calculated on a product-by-product basis on the straight-line method over the estimated economic life of the products not to exceed five years. Amortization of software development costs amounted to $1.4 million, $1.2 million and $1.3 million for the fiscal years ending August 31, 2021, 2020 and 2019, respectively. We expect future amortization expense to vary due to increases in capitalized computer software development costs.
We test capitalized computer software development costs for recoverability whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
Intangible Assets and Goodwill
The Company performs valuations of assets acquired and liabilities assumed on each acquisition accounted for as a business combination and recognizes the assets acquired and liabilities assumed at their acquisition date fair value. Acquired intangible assets include customer relationships, software, trade name, and noncompete agreements. The Company determines the appropriate useful life by performing an analysis of expected cash flows based on historical experience of the acquired businesses. Intangible assets are amortized over their estimated useful lives using the straight-line method, which approximates the pattern in which the majority of the economic benefits are expected to be consumed.
Goodwill represents the excess of the cost of an acquired entity over the fair value of the acquired net assets. Goodwill is not amortized, instead it is tested for impairment annually or when events or circumstances change that would indicate that goodwill might be impaired. Events or circumstances that could trigger an impairment review include, but are not limited to, a significant adverse change in legal factors or in the business climate, an adverse action or assessment by a regulator, unanticipated competition, a loss of key personnel, significant changes in the manner of the Company's use of the acquired assets or the strategy for the Company's overall business, significant negative industry or economic trends or significant under-performance relative to expected historical or projected future results of operations.
Goodwill is tested for impairment at the reporting unit level, which is one level below or the same as an operating segment. As of August 31, 2021, the Company determined that it had four reporting units, Simulations Plus, Cognigen Corporation, DILIsym Services, Inc. and Lixoft. When testing goodwill for impairment, the Company first performs a qualitative assessment to determine whether it is necessary to perform step one of a two-step annual goodwill impairment test for each reporting unit. The Company is required to perform step one only if it concludes that it is more likely than not that a reporting unit's fair value is less than its carrying value. Should this be the case, the first step of the two-step process is to identify whether a potential impairment exists by comparing the estimated fair values of the Company's reporting units with their respective book values, including goodwill. If the estimated fair value of the reporting unit exceeds book value, goodwill is considered not to be impaired, and no additional steps are necessary. If, however, the fair value of the reporting unit is less than book value, then the second step is performed to determine if goodwill is impaired and to measure the amount of impairment loss, if any. The amount of the impairment loss is the excess of the carrying amount of the goodwill over its implied fair value. The estimate of implied fair value of goodwill is primarily based on an estimate of the discounted cash flows expected to result from that reporting unit, but may require valuations of certain internally generated and unrecognized intangible assets such as the Company's software, technology, patents and trademarks. If the carrying amount of goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to the excess.
As of August 31, 2021, the entire balance of goodwill was attributed to three of the Company's reporting units Cognigen, DILIsym and Lixoft. Intangible assets subject to amortization are reviewed for impairment whenever events or circumstances indicate that the carrying amount of these assets may not be recoverable. The Company has not recognized any impairment charges during the periods ended August 31, 2021, 2020 and 2019.
| 49 |
Business Acquisitions
The Company accounted for the acquisition of Cognigen, DILIsym Services Inc., and Lixoft using the purchase method of accounting where the assets acquired and liabilities assumed are recognized based on their respective estimated fair values. The excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill. Determining the fair value of certain acquired assets and liabilities is subjective in nature and often involves the use of significant estimates and assumptions, including, but not limited to, the selection of appropriate valuation methodology, projected revenue, expenses, and cash flows, weighted average cost of capital, discount rates and estimates of terminal values. Business acquisitions are included in the Company's consolidated financial statements as of the date of the acquisition.
Research and Development Costs
Research and development costs are charged to expense as incurred until technological feasibility has been established. These costs include salaries, laboratory experiment, and purchased software that was developed by other companies and incorporated into, or used in the development of, our final products.
Income Taxes
The Company accounts for income taxes in accordance with ASC 740-10, “Income Taxes” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns.
Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each year end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. The provision for income taxes represents the tax payable for the period and the change during the period in deferred tax assets and liabilities.
Stock-Based Compensation
The Company accounts for stock options using the modified prospective method in accordance with FASB ASC 718-10, “Compensation-Stock Compensation”. Under this method, compensation costs include estimated grant date fair value of the awards amortized over the options’ vesting period. Stock-based compensation expense, not including shares issued to Directors for services, was $2.4 million, $1.3 million and $866 thousand for the years ended August 31, 2021, 2020 and 2019, respectively, and is included in the statements of operations as Consulting, Salaries, and Research and Development expense.
ITEM 7A – QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As of August 31, 2021, and August 31, 2020, we had cash and cash equivalents of $37.0 million and $49.2 million, respectively. We hold held-to-maturity short-term investments that are exposed to market risk related to changes in interest rates, which could affect the value of our assets and liabilities. We do not hold any trading and or available-for-sale securities. Some of our cash and cash equivalents are held in money market accounts; however, they are not exposed to market-rate risk.
In the years ended August 31, 2021, 2020, and 2019 we sold $4.8 million, $5.0 million and $4.1 million, respectively, of software licenses through representatives in certain Asian markets in local currencies. As a result, our financial position, results of operations, and cash flows can be affected by fluctuations in foreign currency exchange rates, particularly fluctuations in the yen and RMB exchange rates. These transactions give rise to receivables that are denominated in currencies other than the entity’s functional currency. The value of these receivables is subject to changes because the receivables may become worth more or less due to changes in currency exchange rates. The majority of our software license agreements are denominated in U.S. dollars. We record foreign gains and losses as they are realized. We mitigate our risk from foreign currency fluctuations by adjusting prices in our foreign markets on a periodic basis. We base these changes on market conditions while working closely with our representatives. Our Paris, France, division sells mainly in U.S. dollars and Euros and uses the Euro as a functional currency. As such, we are subject to currency translation and exchange rate changes. We do not hedge currencies or enter into derivative contracts.
| 50 |
ITEM 8 – FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
See the financial statements included elsewhere in this Annual Report beginning at page F-1, which are incorporated herein by reference.
ITEM 9 – CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A – CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our Chief Executive Officer and our Chief Financial Officer, after evaluating our “disclosure controls and procedures” (as defined in Securities Exchange Act of 1934 (the “Exchange Act”) Rules 13a-15(e) and 15d-15(e) as of the end of the period covered by this Annual Report on Form 10-K (the “Evaluation Date”), have concluded that as of the Evaluation Date, our disclosure controls and procedures are effective to ensure that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in Securities and Exchange Commission rules and forms, and to ensure that information required to be disclosed by us in such reports is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, where appropriate, to allow timely decisions regarding required disclosure.
Management Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of consolidated financial statements for external purposes in accordance with U.S. GAAP. Management assessed our internal control over financial reporting as of August 31, 2021, the end of our fiscal year. Management based its assessment on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Management’s assessment included evaluation of elements such as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies, and our overall control environment.
Based on this assessment, management has concluded that our internal control over financial reporting was effective as of the end of the fiscal year to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external reporting purposes in accordance with U.S. GAAP. We reviewed the results of management’s assessment with the Audit Committee of our Board of Directors.
Inherent Limitations on Effectiveness of Controls
Our management, including the CEO and CFO, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well-designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of the effectiveness of controls to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Changes in Internal Control over Financial Reporting
No change in the Company’s internal controls over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) of the Exchange Act) occurred during the Company’s most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B – OTHER INFORMATION
None
| 51 |
PART III
ITEM 10 – DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
Information required by Item 10 is incorporated by reference from the Company’s definitive proxy statement, to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 11 – EXECUTIVE COMPENSATION
The information required by Item 11 is incorporated by reference from the Company’s definitive proxy statement, to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 12 – SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by Item 12 is incorporated by reference from the Company’s definitive proxy statement, to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 13 – CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by Item 13 is incorporated by reference from the Company’s definitive proxy statement, to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 14 – PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by Item 14 is incorporated by reference from the Company’s definitive proxy statement, to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
| 52 |
PART IV
ITEM 15 – EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)
(1) Financial Statements. The consolidated financial statements are included in this Annual Report on Form 10-K beginning on page F-1.
(2) Financial Statement Schedules. All financial statement schedules have been omitted since the information is either not applicable or required or was included in the financial statements or notes included in this Annual Report on Form 10-K.
(3) List of Exhibits required by Item 601 of Regulation S-K. See part (b) below.
(b) Exhibits. The following exhibits are filed or furnished with this report. Those exhibits marked with a (†) refer to management contracts or compensatory plans or arrangements.
| 53 |
__________________________
| ^ | Schedules and exhibits omitted pursuant to Item 601(b)(2) of Registration S-K. The registrant agrees to furnish supplementally a copy of any omitted schedule to the SEC upon request. |
| * | Filed herewith. |
| ** | The XBRL related information in Exhibit 101 shall not be deemed filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to liability of that section and shall not be incorporated by reference into any filing or other document pursuant to the Securities Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing or document. |
| (†) | Refers to management contracts or compensatory plans or arrangements |
| (1) | Incorporated by reference to the Company’s Registration Statement on Form SB-2 (Registration No. 333-6680) filed on March 25, 1997. |
| (2) | Incorporated by reference to an exhibit to the Company’s Form 10-K for the fiscal year ended August 31, 2010. |
| (3) | Incorporated by reference to an exhibit to the Company’s Form 10-Q filed April 9, 2014. |
| (4) | Incorporated by reference to an exhibit to the Company’s Form 8-K/A filed November 18, 2014. |
| (5) | Incorporated by reference to an exhibit to the Company’s Form 8-K filed August 11, 2016. |
| (6) | Incorporated by reference to an exhibit to the Company’s Form 8-K filed August 10, 2016. |
| (7) | Incorporated by reference to an exhibit to the Company’s Form 10-Q filed July 10, 2017. |
| (8) | Incorporated by reference to Appendix A to the Company’s Schedule 14A filed December 29. 2016. |
| (9) | Incorporated by reference to an exhibit to the Company’s Form 8-K filed September 6, 2017. |
| (10) | Incorporated by reference to an exhibit to the Company’s Form 10-K for the fiscal year ended August 31, 2016. |
| (11) | Incorporated by reference to an exhibit to the Company’s Form 10-Q filed July 10, 2018. |
| (12) | Incorporated by reference to an exhibit to the Company’s Form 8-K filed April 2, 2020. |
| (13) | Incorporated by reference to an exhibit to the Company’s Form 8-K filed April 3, 2020. |
| (14) | Incorporated by reference to an exhibit to the Company’s Form 8-K filed September 9, 2020. |
| (15) | Incorporated by reference to Appendix A to the Company’s Definitive Schedule 14A filed December 31, 2018. |
| (16) | Incorporated by reference to an exhibit to the Company’s Form 8-K filed January 4, 2021. |
| (17) | Incorporated by reference to an exhibit to the Company’s Form 10-Q filed January 11, 2021. |
| (18) | Incorporated by reference to an exhibit to the Company’s Form 10-Q filed April 14, 2021. |
| (19) | Incorporated by reference to an exhibit to the Company’s Form 8-K filed June 8, 2021. |
(c) Financial Statement Schedule.
See Item 15(a)(2) above.
| 54 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
October 27, 2021
| SIMULATIONS PLUS, INC. | ||
| By: | /s/ Will Frederick | |
| Will
Fredrick Chief Financial Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | ||
| /s/ Shawn O’Connor | Chief Executive Officer (Principal executive officer) | ||
| Shawn O’Connor | |||
| October 27, 2021 | |||
| /s/ Walter S. Woltosz | Chairman of the Board of Directors | ||
| Walter S. Woltosz | |||
| October 27, 2021 | |||
| /s/ Dr. Lisa LaVange | Director | ||
| Dr. Lisa LaVange | |||
| October 27, 2021 | |||
| /s/ Dr. Daniel Weiner | Director | ||
| Dr. Daniel Weiner | |||
| October 27, 2021 | |||
| /s/ Dr. David L. Ralph | Director | ||
| Dr. David L. Ralph | |||
| October 27, 2021 | |||
| /s/ Dr. John K. Paglia | Director | ||
| Dr. John K. Paglia | |||
| October 27, 2021 | |||
| /s/ Will Frederick | Chief Financial Officer (Principal financial | ||
| Will Frederick | officer and principal accounting officer) | ||
| October 27, 2021 | |||
| 55 |
SIMULATIONS PLUS, INC. & SUBSIDIARY
CONTENTS
August 31, 2021, 2020 and 2019
| Page | |
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | F-2 - F-4 |
| FINANCIAL STATEMENTS | |
| Consolidated Balance Sheets | F-5 |
| Consolidated Statements of Operations and Comprehensive Income | F-6 |
| Consolidated Statements of Shareholders’ Equity | F-7 |
| Consolidated Statements of Cash Flows | F-8 |
| Notes to Consolidated Financial Statements | F-9 – F-34 |
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Simulations Plus, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Simulations Plus, Inc. and Subsidiaries (the Company) as of August 31, 2021, and 2020, and the related consolidated statements of operations and comprehensive income, stockholders’ equity, and cash flows for each of the years in the three-year period ended August 31, 2021, and the related notes (collectively referred to as the financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of August 31, 2021, and 2020, and the results of its operations and its cash flows for each of the years in the three-year period ended August 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of August 31, 2021, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated October 27, 2021, expressed an unqualified opinion.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Revenue Recognition – Contract cost estimates
Description of the Matter
As discussed in Note 2 and Note 3 to the Consolidated Financial Statements, the Company earns a portion of its revenue through consulting service agreements. For performance obligations related to services that are required to be recognized over time, the Company generally measures its progress to completion using an input measure of total labor costs incurred divided by total labor costs expected to be incurred.
| F-2 |
Auditing revenue recognition is complex and highly judgmental due to the variability and uncertainty associated with the Company’s assessment of measure of progress. Changes in these estimates would have a significant effect on the amount of revenue recognized.
How We Addressed the Matter in Our Audit
We obtained an understanding, evaluated the design, and tested the operating effectiveness of controls that address the risk of material misstatement of consulting services revenue including those associated with cost to complete estimates. We tested controls over management’s process to collect, review, and approve the data used in assessing revenue recognized over time.
To test the measures of progress used for performance obligations related to services that are required to be recognized over time, our audit procedures included, among others, evaluating the appropriateness of the Company’s accounting policy for each type of arrangement, testing the identified measure of performance by reading contracts with customers, including all amendments, and reviewing the contract analyses prepared by management. We evaluated whether the selected measures of progress towards satisfaction of performance obligations were applied consistently. We also tested the completeness and accuracy of the underlying data used for the measure of progress by testing the underlying cost data.
|
Rose, Snyder & Jacobs LLP |
||
| We have served as the Company’s auditor since 2004. | ||
| Encino, California | ||
| October 27, 2021 | ||
| F-3 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Simulations Plus, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Simulations Plus, Inc. and Subsidiaries (the Company’s) internal control over financial reporting as of August 31, 2021, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of August 31, 2021, based on criteria established in Internal Control—Integrated Framework (2013) issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheet as of August 31, 2021, and the related consolidated statements of operations and comprehensive income, stockholders’ equity, and cash flows for each of the three years in the period ended August 31, 2021, and related notes, and our report dated October 27, 2021, expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Rose, Snyder & Jacobs LLP
Encino, CA
October 27, 2021
| F-4 |
SIMULATIONS PLUS, INC.
CONSOLIDATED BALANCE SHEETS
| August 31, | ||||||||
| (in thousands, except share and per share amounts) | 2021 | 2020 | ||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Accounts receivable, net of allowance for doubtful accounts of $ | ||||||||
| Revenues in excess of billings | ||||||||
| Prepaid income taxes | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Short-term investments | ||||||||
| Total current assets | ||||||||
| Long-term assets | ||||||||
| Capitalized computer software development costs, net of accumulated amortization of $ | ||||||||
| Property and equipment, net | ||||||||
| Operating lease right of use asset | ||||||||
| Intellectual property, net of accumulated amortization of $ | ||||||||
| Other intangible assets, net of accumulated amortization of $ | ||||||||
| Goodwill | ||||||||
| Other assets | ||||||||
| Total assets | $ | $ | ||||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued payroll and other expenses | ||||||||
| Contracts payable - current portion | ||||||||
| Billings in excess of revenues | ||||||||
| Operating lease liability - current portion | ||||||||
| Deferred revenue | ||||||||
| Total current liabilities | ||||||||
| Long-term liabilities | ||||||||
| Deferred income taxes, net | ||||||||
| Operating lease liability | ||||||||
| Contracts payable – net of current portion | ||||||||
| Total liabilities | ||||||||
| Commitments and contingencies | ||||||||
| Shareholders' equity | ||||||||
| Preferred stock, $ par value shares authorized shares issued and outstanding | $ | $ | ||||||
| Common stock, $ par value and additional paid-in capital — shares authorized, and shares issued and outstanding | ||||||||
| Retained earnings | ||||||||
| Accumulated other comprehensive income (loss) | ( | ) | ||||||
| Total shareholders' equity | ||||||||
| Total liabilities and shareholders' equity | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
SIMULATIONS PLUS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
| Years Ended August 31, | ||||||||||||
| (in thousands, except per common share amounts) | 2021 | 2020 | 2019 | |||||||||
| Revenues | $ | $ | $ | |||||||||
| Cost of revenues | ||||||||||||
| Gross profit | ||||||||||||
| Operating expenses | ||||||||||||
| Research and development | ||||||||||||
| Selling, general and administrative | ||||||||||||
| Total operating expenses | ||||||||||||
| Income from operations | ||||||||||||
| Other income (expense) | ||||||||||||
| Interest income | ||||||||||||
| Interest expense | ( | ) | ||||||||||
| Change in value of contingent consideration | ( | ) | ( | ) | ( | ) | ||||||
| Gain (loss) on currency exchange | ( | ) | ( | ) | ||||||||
| Total other income (expense), net | ( | ) | ( | ) | ( | ) | ||||||
| Income before income taxes | ||||||||||||
| Provision for income taxes | ( | ) | ( | ) | ( | ) | ||||||
| Net Income | $ | $ | $ | |||||||||
| Earnings per share | ||||||||||||
| Basic | $ | $ | $ | |||||||||
| Diluted | $ | $ | $ | |||||||||
| Weighted-average common shares outstanding | ||||||||||||
| Basic | ||||||||||||
| Diluted | ||||||||||||
| Other comprehensive income (loss), net of tax | ||||||||||||
| Foreign currency translation adjustments | ( | ) | ||||||||||
| Comprehensive income | $ | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
SIMULATIONS PLUS, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY
Year ended August 31, | ||||||||||||
| (in thousands, except per common share amounts) | 2021 | 2020 | 2019 | |||||||||
| Common stock and additional paid in capital | ||||||||||||
| Balance, beginning of period | $ | $ | $ | |||||||||
| Exercise of stock options | ||||||||||||
| Stock-based compensation | ||||||||||||
| Shares issued to Directors for services | ||||||||||||
| Shares issued - Lixoft | ||||||||||||
| Common stock issued for cash, net | ||||||||||||
| Balance, end of period | ||||||||||||
| Retained earnings | ||||||||||||
| Balance, beginning of period | ||||||||||||
| Cumulative effect of changes related to adoption of ASC 606 | ( | ) | ||||||||||
| Declaration of dividends | ( | ) | ( | ) | ( | ) | ||||||
| Net income | ||||||||||||
| Balance, end of period | ||||||||||||
| Accumulated other comprehensive income | ||||||||||||
| Balance, beginning of period | ||||||||||||
| Other comprehensive income (loss) | ( | ) | ||||||||||
| Balance, end of period | ( | ) | ||||||||||
| Total shareholders’ equity | $ | $ | $ | |||||||||
| Cash dividends declared per common share | $ | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-7 |
SIMULATIONS PLUS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year ended August 31, | ||||||||||||
| (in thousands) | 2021 | 2020 | 2019 | |||||||||
| Cash flows from operating activities | ||||||||||||
| Net income | $ | $ | $ | |||||||||
| Adjustments to reconcile net income to net cash provided by operating activities | ||||||||||||
| Depreciation and amortization | ||||||||||||
| Change in value of contingent consideration | ||||||||||||
| Amortization of investment premiums | ||||||||||||
| Stock-based compensation | ||||||||||||
| Deferred income taxes | ( | ) | ( | ) | ( | ) | ||||||
| Currency translation adjustments | ( | ) | ||||||||||
| (Increase) decrease in | ||||||||||||
| Accounts receivable | ( | ) | ( | ) | ||||||||
| Revenues in excess of billings | ( | ) | ( | ) | ||||||||
| Prepaid income taxes | ( | ) | ( | ) | ( | ) | ||||||
| Prepaid expenses and other assets | ( | ) | ( | ) | ( | ) | ||||||
| Increase (decrease) in | ||||||||||||
| Accounts payable | ( | ) | ||||||||||
| Accrued payroll and other expenses | ||||||||||||
| Billings in excess of revenues | ( | ) | ( | ) | ||||||||
| Deferred revenue | ( | ) | ( | ) | ||||||||
| Net cash provided by operating activities | ||||||||||||
| Cash flows from investing activities | ||||||||||||
| Purchases of property and equipment | ( | ) | ( | ) | ( | ) | ||||||
| Purchases of intellectual property | ( | ) | ||||||||||
| Purchase of short-term investments | ( | ) | ( | ) | ||||||||
| Proceeds from sale of short-term investments | ||||||||||||
| Cash used to acquire subsidiaries | ( | ) | ||||||||||
| Cash received in acquisition | ||||||||||||
| Capitalized computer software development costs | ( | ) | ( | ) | ( | ) | ||||||
| Net cash used in investing activities | ( | ) | ( | ) | ( | ) | ||||||
| Cash flows from financing activities | ||||||||||||
| Payment of dividends | ( | ) | ( | ) | ( | ) | ||||||
| Payments on contracts payable | ( | ) | ( | ) | ( | ) | ||||||
| Proceeds from the exercise of stock options | ||||||||||||
| Proceeds from follow-on public offering, net | ||||||||||||
| Net cash provided by (used in) financing activities | ( | ) | ( | ) | ||||||||
| Net increase (decrease) in cash and cash equivalents | ( | ) | ||||||||||
| Cash and cash equivalents, beginning of year | ||||||||||||
| Cash and cash equivalents, end of period | $ | $ | $ | |||||||||
| Supplemental disclosures of cash flow information | ||||||||||||
| Income taxes paid | $ | $ | $ | |||||||||
| Non-Cash Investing and Financing Activities | ||||||||||||
| Stock issued for acquisition of Lixoft | $ | $ | $ | |||||||||
| Creation of contract liabilities for acquisition of subsidiaries | $ | $ | $ | |||||||||
| Right of use assets capitalized | $ | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-8 |
Simulations Plus, Inc.
Notes to Consolidated Financial Statements
For the Year Ended August 31, 2021
NOTE 1 – ORGANIZATION AND LINES OF BUSINESS
Organization
Simulations Plus, Inc. (“The Company”) was incorporated on July 17, 1996. In September 2014, Simulations Plus acquired all of the outstanding equity interests of Cognigen Corporation (“Cognigen”) and Cognigen became a wholly-owned subsidiary of Simulations Plus, Inc. In June 2017, Simulations Plus acquired DILIsym Services, Inc. (“DILIsym”) as a wholly-owned subsidiary. In April 2020, Simulations Plus, Inc. acquired Lixoft, a French société par actions simplifiée (“Lixoft) as a wholly-owned subsidiary pursuant to a stock purchase and contribution agreement. (Collectively, “Company”, “we”, “us”, “our”).
Effective September 1, 2021, the Company merged both Cognigen Corporation and DILIsym, Services, Inc. with and into Simulations Plus, Inc. through short-form mergers (the “Mergers”). To effectuate the Mergers, the Company filed Certificates of Ownership with the Secretaries of State of the states of Delaware (Cognigen’s and DILIsym’s state of incorporation) and California (the Company’s state of incorporation). Consummation of the Mergers was not subject to approval of the Company’s stockholders and did not impact the rights of the Company’s stockholders.
Lines of Business
We are a premier developer of drug discovery and development software for modeling and simulation, and for the prediction of molecular properties utilizing both artificial intelligence (“AI”) as well as machine learning based technology. We also provide consulting services ranging from early drug discovery through preclinical and clinical trial data analysis and for submissions to regulatory agencies. Our software and consulting services are provided to major pharmaceutical, biotechnology, agrochemical, cosmetics and food industry companies, and to regulatory agencies worldwide for use in the conduct of industry-based research.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements include the accounts of Simulations Plus and, as of September 2, 2014, its wholly-owned subsidiary, Cognigen, as of June 1, 2017, the accounts of DILIsym, and as of April 1, 2020, Lixoft. All significant intercompany accounts and transactions are eliminated in consolidation.
Use of Estimates
Our financial statements and accompanying notes are prepared in accordance with accounting principles generally accepted in the United States of America. Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. These estimates and assumptions are affected by management’s application of accounting policies. Actual results could differ from those estimates. Significant accounting policies for us include revenue recognition, accounting for capitalized computer software development costs, valuation of stock options, and accounting for income taxes.
Reclassifications
Certain numbers in the prior year have been reclassified to conform to the current year's presentation.
Revenue Recognition
We generate revenue primarily from the sale of software licenses and providing consulting services to the pharmaceutical industry for drug development.
| F-9 |
The Company determines revenue recognition through the following steps:
| i. | Identification of the contract, or contracts, with a customer |
| ii. | Identification of the performance obligations in the contract |
| iii. | Determination of the transaction price |
| iv. | Allocation of the transaction price to the performance obligations in the contract |
| v. | Recognition of revenue when, or as, the Company satisfies a performance obligation |
Deferred Commissions
Sales commissions earned by our sales force and our commissioned sales representatives are considered incremental and recoverable costs of obtaining a contract with a customer. Sales commissions for new contracts are deferred and then amortized on a straight-line basis over a period of benefit. We determined the period of benefit by taking into consideration our customer contracts, our technology and other factors. Sales commissions for renewal contracts are deferred and then amortized on a straight-line basis over the related contractual renewal period. Amortization expense is included in sales and marketing expenses on the consolidated statements of operations and comprehensive income as Selling, general, and administrative expense.
Practical Expedients and Exemptions
The Company has elected the following additional practical expedients in applying Topic 606:
| · | Commission Expense: We apply the practical expedient in ASC Topic 606 to expense costs as incurred for sales commissions when the period of benefit is one year or less. Most of our contracts are of a duration of one year or less, few, if any of the longer-term contracts have commissions associated with them. This expense is included in the consolidated statements of operations and comprehensive income as Selling, general, and administrative expense. |
| · |
Transaction Price Allocated to Future Performance Obligations
ASC 606 requires that the Company disclose the aggregate amount of transaction price that is allocated to performance obligations that have not yet been satisfied as of August 31, 2021. ASC 606 provides certain practical expedients that limit the requirement to disclose the aggregate amount of transaction price allocated to unsatisfied performance obligations.
The Company applied the practical expedient to not disclose the amount of transaction price allocated to unsatisfied performance obligations when the performance obligation is part of a contract that has an original expected duration of one year or less. |
Cash and Cash Equivalents
For purposes of the statements of cash flows, the Company considers all highly liquid investments purchased with original maturities of three months or less to be cash equivalents.
Accounts Receivable
We analyze the age of customer balances, historical bad-debt experience, customer creditworthiness, and changes in customer payment terms when making estimates of the collectability of the Company’s trade accounts receivable balances. If we determine that the financial conditions of any of our customers have deteriorated, whether due to customer-specific or general economic issues, an increase in the allowance may be made. Accounts receivable are written off when reasonable collection attempts have failed.
| F-10 |
Investments
We may invest excess cash balances in short-term and long-term marketable debt securities. Investments may consist of certificates of deposit, money market accounts, government-sponsored enterprise securities, corporate bonds and/or commercial paper. The Company accounts for its investment in marketable securities in accordance with FASB ASC 320, Investments – Debt and Equity Securities. This statement requires debt securities to be classified into three categories:
Held-to-maturity—Debt securities that the entity has the positive intent and ability to hold to maturity are reported at amortized cost. Discounts and premiums to par value of the debt securities are amortized to interest income/expense over the term of the security. No gains or losses on investment securities are realized until they are sold or a decline in fair value is determined to be other-than-temporary.
Trading Securities—Debt securities that are bought and held primarily for the purpose of selling in the near term are reported at fair value, with unrealized gains and losses included in earnings.
Available-for-Sale—Debt securities not classified as either securities held-to-maturity or trading securities are reported at fair value with unrealized gains or losses excluded from earnings and reported as a separate component of shareholders’ equity.
We classify our investments in marketable debt securities based on the facts and circumstances present at the time of purchase of the securities. During the year ended August 31, 2021, all of our investments were classified as held-to-maturity.
Held-to-maturity investments are measured and recorded at amortized cost on the Company’s Consolidated Balance Sheets. Discounts and premiums to par value of the debt securities are amortized to interest income/expense over the term of the security. No gains or losses on investment securities are realized until they are sold or a decline in fair value is determined to be other-than-temporary.
Capitalized Computer Software Development Costs
Software development costs are capitalized in accordance with ASC 985-20, “Costs of Software to Be Sold, Leased, or Marketed”. Capitalization of software development costs begins upon the establishment of technological feasibility and is discontinued when the product is available for sale.
The establishment of technological feasibility and the ongoing assessment for recoverability of capitalized software development costs require considerable judgment by management with respect to certain external factors including, but not limited to, technological feasibility, anticipated future gross revenues, estimated economic life, and changes in software and hardware technologies. Capitalized computer software development costs are comprised primarily of salaries and direct payroll-related costs and the purchase of existing software to be used in the Company's software products.
Amortization of capitalized computer software
development costs is provided on a product-by-product basis on the straight-line method over the estimated economic life of the products
(not to exceed five years). Amortization of software development costs amounted to $
We test capitalized computer software development costs for recoverability whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
Property and Equipment
Property and equipment are recorded at cost, or fair market value for property and equipment acquired in business combinations, less accumulated depreciation and amortization. Depreciation and amortization are provided using the straight-line method over the estimated useful lives as follows:
| Equipment | ||
| Computer equipment | ||
| Furniture and fixtures | ||
| Leasehold improvements |
Maintenance and minor replacements are charged to expense as incurred. Gains and losses on disposals are included in the results of operations.
| F-11 |
Internal-use Software
We have a service contract related to the implementation of internally used software. In accordance with ASC 350-40 “Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract”, we have capitalized certain internal-use software which are included in long-term assets.
The amortization will be classified as Selling, general, and administrative expenses on the consolidated statement of operations and comprehensive income and maintenance and minor upgrades are charged to expense as incurred. Gains and losses on disposals are included in the results of operations. No amortization has been expensed for the project as it is still in progress.
Leases
We determine if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets and operating lease liabilities (current and long-term) in our consolidated balance sheets.
ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of our leases do not provide an implicit rate, we generally use our incremental borrowing rate based on the estimated rate of interest for collateralized borrowing over a similar term of the lease payments at commencement date. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. Our lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
Supplemental balance sheet information related to operating leases was as follows as of August 31, 2021:
| (in thousands) | ||||
| Right of use assets | $ | |||
| Lease Liabilities, Current | $ | |||
| Lease Liabilities, Long-term | $ | |||
| Operating lease costs | $ | |||
| Weighted Average remaining lease term | ||||
| Weighted Average Discount rate | ||||
Intangible Assets and Goodwill
The Company performs valuations of assets acquired and liabilities assumed on each acquisition accounted for as a business combination and recognizes the assets acquired and liabilities assumed at their acquisition-date fair value. Acquired intangible assets include customer relationships, software, trade names, and noncompete agreements. We determine the appropriate useful life by performing an analysis of expected cash flows based on historical experience of the acquired businesses. Intangible assets are amortized over their estimated useful lives using the straight-line method, which approximates the pattern in which the majority of the economic benefits are expected to be consumed.
Goodwill represents the excess of the cost of an acquired entity over the fair value of the acquired net assets. Goodwill is not amortized, instead it is tested for impairment annually or when events or circumstances change that would indicate that goodwill might be impaired. Events or circumstances that could trigger an impairment review include, but are not limited to, a significant adverse change in legal factors or in the business climate, an adverse action or assessment by a regulator, unanticipated competition, a loss of key personnel, significant changes in the manner of our use of the acquired assets or the strategy for our overall business, significant negative industry or economic trends, or significant underperformance relative to expected historical or projected future results of operations.
| F-12 |
Goodwill is tested for impairment at the reporting unit level, which is one level below or the same as an operating segment. As of August 31, 2021, the Company determined that it has four reporting units, Simulations Plus, Cognigen Corporation, DILIsym Services, Inc. and Lixoft. When testing goodwill for impairment, the Company first performs a qualitative assessment to determine whether it is necessary to perform step one of a two-step annual goodwill impairment test for each reporting unit. The Company is required to perform step one only if it concludes that it is more likely than not that a reporting unit's fair value is less than its carrying value. Should this be the case, the first step of the two-step process is to identify whether a potential impairment exists by comparing the estimated fair values of the Company's reporting units with their respective book values, including goodwill. If the estimated fair value of the reporting unit exceeds book value, goodwill is considered not to be impaired, and no additional steps are necessary. If, however, the fair value of the reporting unit is less than book value, then the second step is performed to determine if goodwill is impaired and to measure the amount of impairment loss, if any. The amount of the impairment loss is the excess of the carrying amount of the goodwill over its implied fair value. The estimate of implied fair value of goodwill is primarily based on an estimate of the discounted cash flows expected to result from that reporting unit, but may require valuations of certain internally generated and unrecognized intangible assets such as the Company's software, technology, patents and trademarks. If the carrying amount of goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to the excess.
As of August 31, 2021, the entire balance of goodwill
was attributed to three of the Company's reporting units, Cognigen Corporation, DILIsym Services, Inc. and Lixoft. Intangible assets subject
to amortization are reviewed for impairment whenever events or circumstances indicate that the carrying amount of these assets may not
be recoverable. The Company has
Reconciliation of Goodwill as of August 31, 2021, and 2020:
| (in thousands) | Cognigen | DILIsym | Lixoft | Total | ||||||||||||
| Balance, August 31, 2019 | $ | $ | $ | $ | ||||||||||||
| Addition | ||||||||||||||||
| Impairments | ||||||||||||||||
| Balance, August 31, 2020 | ||||||||||||||||
| Addition | ||||||||||||||||
| Impairments | ||||||||||||||||
| Balance, August 31, 2021 | $ | $ | $ | $ | ||||||||||||
Other Intangible Assets
The following table summarizes other intangible assets as of August 31, 2021:
| (in thousands) | Amortization Period | Acquisition Value | Accumulated Amortization | Net book value | ||||||||||
| Cognigen | ||||||||||||||
| Customer relationships | $ | $ | $ | |||||||||||
| Trade Name | ||||||||||||||
| Covenants not to compete | ||||||||||||||
| DILIsym | ||||||||||||||
| Customer relationships | ||||||||||||||
| Trade Name | ||||||||||||||
| Covenants not to compete | ||||||||||||||
| Lixoft | ||||||||||||||
| Customer relationships | ||||||||||||||
| Trade Name | ||||||||||||||
| Covenants not to compete | ||||||||||||||
| $ | $ | $ | ||||||||||||
| F-13 |
The following table summarizes other intangible assets as of August 31, 2020:
| (in thousands) | Amortization Period | Acquisition Value | Accumulated Amortization | Net book value | ||||||||||
| Cognigen | ||||||||||||||
| Customer relationships | $ | $ | $ | |||||||||||
| Trade Name | ||||||||||||||
| Covenants not to compete | ||||||||||||||
| DILIsym | ||||||||||||||
| Customer relationships | ||||||||||||||
| Trade Name | ||||||||||||||
| Covenants not to compete | ||||||||||||||
| Lixoft | ||||||||||||||
| Customer relationships | ||||||||||||||
| Trade Name | ||||||||||||||
| Covenants not to compete | ||||||||||||||
| $ | $ | $ | ||||||||||||
Total amortization expense for the years ended
August 31, 2021, 2020 and 2019 was $
Future amortization of intangible assets for the next five years is as follows:
| (in thousands) | ||||||
Year ending August 31, | Amount | |||||
| 2022 | $ | |||||
| 2023 | $ | |||||
| 2024 | $ | |||||
| 2025 | $ | |||||
| 2026 | $ | |||||
Business Acquisitions
The Company accounted for the acquisition of Cognigen, DILIsym, and Lixoft using the purchase method of accounting where the assets acquired and liabilities assumed are recognized based on their respective estimated fair values. The excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill. Determining the fair value of certain acquired assets and liabilities is subjective in nature and often involves the use of significant estimates and assumptions, including, but not limited to, the selection of appropriate valuation methodology, projected revenue, expenses and cash flows, weighted average cost of capital, discount rates, estimates of advertiser and publisher turnover rates and estimates of terminal values. Business acquisitions are included in the Company's consolidated financial statements as of the date of the acquisition.
| F-14 |
Fair Value of Financial Instruments
Assets and liabilities recorded at fair value in the Consolidated Balance Sheets are categorized based upon the level of judgment associated with the inputs used to measure their fair value. The categories, as defined by the standard are as follows:
| Level Input: | Input Definition: | |
| Level I | Inputs are unadjusted, quoted prices for identical assets or liabilities in active markets at the measurement date. | |
| Level II | Inputs, other than quoted prices included in Level I, that are observable for the asset or liability through corroboration with market data at the measurement date. | |
| Level III | Unobservable inputs that reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. |
For certain of our financial instruments, including accounts receivable, accounts payable, and accrued payroll and other expenses, the carrying amounts approximate fair value due to their short-term nature.
The following table summarizes fair value measurements as of August 31, 2021, and August 31, 2020, for assets and liabilities measured at fair value on a recurring basis:
| August 31, 2021 | ||||||||||||||||
| (in thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| Cash and cash equivalents | $ | $ | $ | $ | ||||||||||||
| Short-term investments | $ | $ | $ | $ | ||||||||||||
| Acquisition-related contingent consideration obligations | $ | $ | $ | $ | ||||||||||||
| August 31, 2020 | ||||||||||||||||
| (in thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| Cash and cash equivalents | $ | $ | $ | $ | ||||||||||||
| Short-term investments | $ | $ | $ | $ | ||||||||||||
| Acquisition-related contingent consideration obligations | $ | $ | $ | $ | ||||||||||||
As of August 31, 2021, and 2020, the Company had a liability for contingent consideration related to its acquisition of Lixoft and DILIsym. The fair value measurement of the contingent consideration obligations is determined using Level 3 inputs. The fair value of contingent consideration obligations is based on a discounted cash flow model using a probability-weighted income approach. These fair value measurements represent Level 3 measurements as they are based on significant inputs not observable in the market. Significant judgment is employed in determining the appropriateness of these assumptions as of the acquisition date and for each subsequent period. Accordingly, changes in assumptions could have a material impact on the amount of contingent consideration expense the Company records in any given period. Changes in the value of the contingent consideration obligations are recorded in the Company’s Consolidated Statement of Operations.
The following is a reconciliation of contingent consideration value:
| (in thousands) | ||||
| Value as of August 31, 2020 | $ | |||
| Contingent consideration payments | ( | ) | ||
| Change in value of contingent consideration | ||||
| Value as of August 31, 2021 | $ | |||
| F-15 |
Marketing
The Company expenses marketing and advertising
costs as incurred. Marketing costs for the years ended August 31, 2021, 2020 and 2019 were approximately $
Research and Development Costs
Research and development costs are charged to expense as incurred until technological feasibility has been established. These costs include salaries, laboratory experiment, and purchased software which was developed by other companies and incorporated into, or used in the development of, our final products.
Income Taxes
The Company accounts for income taxes in accordance with ASC 740-10, “Income Taxes” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns.
Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each year-end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. The provision for income taxes represents the tax payable for the period and the change during the period in deferred tax assets and liabilities.
Intellectual property
On February 28, 2012, we bought out the royalty
agreement with Enslein Research. The cost of $
On May 15, 2014, we entered into a termination
and non-assertion agreement with TSRL, Inc., pursuant to which the parties agreed to terminate an exclusive software licensing agreement
entered into between the parties in 1997. As a result, the Company obtained a perpetual right to use certain source code and data, and
TSRL relinquished any rights and claims to any GastroPlus products and to any claims to royalties or other payments under that 1997 agreement.
We agreed to pay TSRL total consideration of $
On June 1, 2017, as part of the acquisition of
DILIsym the Company acquired certain developed technologies associated with the drug induced liver disease (DILI). These technologies
were valued at approximately $
In September 2018, we purchased certain intellectual
property rights of Entelos Holding Company. The cost of $
On April 1, 2020, as part of the acquisition of
Lixoft, the Company acquired certain developed technologies associated with the Lixoft scientific software. These technologies were valued
at approximately $
| F-16 |
The following table summarizes intellectual property as of August 31, 2021:
| (in thousands) | Amortization Period | Acquisition Value | Accumulated Amortization | Net Book Value | ||||||||||
| Royalty Agreement buy out-Enslein Research | $ | $ | $ | |||||||||||
| Termination/nonassertion agreement-TSRL Inc. | ||||||||||||||
| Developed technologies–DILIsym acquisition | ||||||||||||||
| Intellectual rights of Entelos Holding Company | ||||||||||||||
| Developed technologies–Lixoft acquisition | ||||||||||||||
| $ | $ | $ | ||||||||||||
The following table summarizes intellectual property as of August 31, 2020:
| (in thousands) | Amortization Period | Acquisition Value | Accumulated Amortization | Net Book Value | ||||||||||
| Royalty Agreement buy out-Enslein Research | $ | $ | $ | |||||||||||
| Termination/nonassertion agreement-TSRL Inc. | ||||||||||||||
| Developed technologies–DILIsym acquisition | ||||||||||||||
| Intellectual rights of Entelos Holding Company | ||||||||||||||
| Developed technologies–Lixoft acquisition | ||||||||||||||
| $ | $ | $ | ||||||||||||
Total amortization expense for intellectual property
agreements for the years ended August 31, 2021, 2020 and 2019 was $
Future amortization of intellectual property for the next five years is as follows:
| (in thousands) | ||||
Year ending August 31, | Amount | |||
| 2022 | $ | |||
| 2023 | $ | |||
| 2024 | $ | |||
| 2025 | $ | |||
| 2026 | $ | |||
| F-17 |
The Company reports earnings per share in accordance with FASB ACS 260-10. Basic earnings per share is computed by dividing income available to common shareholders by the weighted-average number of common shares available. Diluted earnings per share is computed similarly to basic earnings per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. The components of basic and diluted earnings per share for the years ended August 31, 2021, 2020 and 2019 were as follows:
| August 31, | ||||||||||||
| (in thousands) | 2021 | 2020 | 2019 | |||||||||
| Numerator | ||||||||||||
| Net income attributable to common shareholders | $ | $ | $ | |||||||||
| Denominator | ||||||||||||
| Weighted-average number of common shares outstanding during the year | ||||||||||||
| Dilutive effect of stock options | ||||||||||||
| Common stock and common stock equivalents used for diluted earnings per share | ||||||||||||
Compensation costs related to stock options are determined in accordance with FASB ASC 718-10, “Compensation-Stock Compensation”, using the modified prospective method. Under this method, compensation cost is calculated based on the grant-date fair value estimated in accordance with FASB ASC 718-10, amortized on a straight-line basis over the options’ vesting period. Stock-based compensation expense related to stock options, not including shares issued to Directors for services, was $million, $ million and $ thousand for the years ended August 31, 2021, 2020 and 2019, respectively. This expense is included in the consolidated statements of operations and comprehensive income as selling, general, and administration and research and development expense.
Impairment of Long-lived Assets
The Company accounts for the impairment and disposition
of long-lived assets in accordance with ASC 350, “Intangibles – Goodwill and Other” and ASC 360, “Property
and Equipment”. Long-lived assets to be held and used are reviewed for events or changes in circumstances that indicate that
their carrying value may not be recoverable. We measure recoverability by comparing the carrying amount of an asset to the expected future
undiscounted net cash flows generated by the asset. If we determine that the asset may not be recoverable, or if the carrying amount of
an asset exceeds its estimated future undiscounted cash flows, we recognize an impairment charge to the extent of the difference between
the fair value and the asset's carrying amount.
Recently Issued Accounting Standards
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes existing guidance on accounting for leases in "Leases (Topic 840)" and generally requires all leases to be recognized in the consolidated balance sheet. ASU 2016-02 is effective for annual and interim reporting periods beginning after December 15, 2018. The Company adopted this ASU on September 1, 2019.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which is intended to simplify various areas related to the accounting for income taxes and improve consistent application of Topic 740. The guidance eliminates certain exceptions related to the approach for intra-period tax allocation, the methodology for calculating income taxes in an interim period, and the recognition of deferred tax liabilities for outside basis differences related to changes in ownership of equity method investments and foreign subsidiaries. The guidance also simplifies aspects of accounting for franchise taxes and the accounting for the enacted changes in tax laws or rates, as well as the accounting for the step-up in the tax basis of goodwill. ASU 2019-12 is effective for us beginning in fiscal 2022; The adoption of the new standard is not expected to have a material impact on the Company’s consolidated financial statements.
| F-18 |
In March 2020, the FASB issued Accounting Standards Update (“ASU”) 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting (“ASU 2020-04”). The amendments in ASU 2020-04 provide temporary optional expedients and exceptions for applying GAAP to contract modifications, hedging relationships and other transactions to ease the potential accounting and financial reporting burden associated with transitioning away from reference rates that are expected to be discontinued, including the London Interbank Offered Rate (“LIBOR”). This ASU is effective as of March 12, 2020, through December 31, 2022. The adoption of the new standard is not expected to have a material impact on our financial statements or related disclosures.
NOTE 3 – REVENUE RECOGNITION
We generate revenue primarily from the sale of software licenses and providing consulting services to the pharmaceutical industry for drug development.
The Company determines revenue recognition through the following steps:
| i. | Identification of the contract, or contracts, with a customer |
| ii. | Identification of the performance obligations in the contract |
| iii. | Determination of the transaction price |
| iv. | Allocation of the transaction price to the performance obligations in the contract |
| v. | Recognition of revenue when, or as, the Company satisfies a performance obligation |
Components of Revenue
The following is a description of principal activities from which the Company generates revenue. As part of the accounting for these arrangements, the Company must develop assumptions that require judgment to determine the stand-alone selling price for each performance obligation identified in the contract. Stand-alone selling prices are determined based on the prices at which the Company separately sells its services or goods.
| Revenue Components | Typical Payment Terms | |
| Software Revenues: | ||
|
Software revenues are generated primarily from sales of software licenses at the time the software is unlocked and the term commences. The license period typically is one year or less. Along with the license a di minimis amount of customer support is provided to assist the customer with the software. Should the customer need more than a di minimis amount of support they can choose to enter into a separate contract for additional training. Most software is installed on our customers’ servers and the Company has no control of the software once the sale is made.
For certain software arrangements the Company hosts the licenses on servers maintained by the Company, Revenue for those arrangements are accounted as Software as a Service over the life of the contract. These arrangements are a small portion of software revenues of the Company. |
Payments are generally due upon invoicing on a net 30 basis unless other payment terms are negotiated with the customer based on customer history. Typical industry standards apply. | |
| Consulting Contracts: | ||
| Consulting services provided to our customers are generally recognized over time as the contracts are performed and the services are rendered. The company measures its consulting revenue based on time expended compared to total estimated hours to complete a project. The Company believes the methods chosen for its contract revenue best depicts the transfer of benefits to the customer under the contracts. | Payment terms vary, depending on the size of the contract, credit history and history with the client and deliverables within the contract. |
| Consortium Member Based Services: | ||
| The performance obligation is recognized on a time elapsed basis, by month, for which the services are provided, as the Company transfers control evenly over the contractual period. | Payment is due at the beginning of the period, generally on a net 30 or 60 basis. |
| F-19 |
Remaining performance obligations that do not fall under the expedients, require the Company to perform various consulting and software development services and consortium memberships of approximately $6.2 million. It is anticipated these revenues will be recognized within the next year.
Contract Liabilities
During the year ended August 31, 2021, the Company recognized $430 thousand of revenue that was included in contract liabilities as of August 31, 2020.
Disaggregation of Revenues
The components of disaggregation of revenue for the years ended August 31, 2021, 2020 and 2019 were as follows:
| Year ended August 31, | ||||||||||||
| (in thousands) | 2021 | 2020 | 2019 | |||||||||
| Software licenses | ||||||||||||
| Point in time | $ | $ | $ | |||||||||
| Over time | ||||||||||||
| Consulting services | ||||||||||||
| Over time | ||||||||||||
| Total revenue | $ | $ | $ | |||||||||
Contracts in Progress
Contracts in progress are included in the accompanying balance sheets under the following captions:
| Year ended August 31, | ||||||||||||
| (in thousands) | 2021 | 2020 | 2019 | |||||||||
| Revenues in excess of billings | $ | $ | $ | |||||||||
| Billings in excess of revenues | ( | ) | ( | ) | ( | ) | ||||||
| Revenues over billings on uncompleted contracts | $ | $ | $ | |||||||||
Cost, estimated earnings, and billings on uncompleted contracts are summarized as follows as of August 31, 2021, 2020 and 2019:
| August 31, | ||||||||||||
| (in thousands) | 2021 | 2020 | 2019 | |||||||||
| Revenues earned to date on uncompleted contracts | $ | $ | $ | |||||||||
| Billings to date on uncompleted contracts | ( | ) | ( | ) | ( | ) | ||||||
| Revenues over billings on uncompleted contracts | $ | $ | $ | |||||||||
Balance increases and decreases in these accounts are due to the timing of amounts billed, payments received, and revenue recognized.
| F-20 |
NOTE 4 – PROPERTY AND EQUIPMENT
Property and equipment consisted of the following:
| August 31, | ||||||||
| (in thousands) | 2021 | 2020 | ||||||
| Equipment | $ | $ | ||||||
| Computer equipment | ||||||||
| Furniture and fixtures | ||||||||
| Leasehold improvements | ||||||||
| Construction in progress | ||||||||
| Subtotal | ||||||||
| Less accumulated depreciation and amortization | ( | ) | ( | ) | ||||
| Total | $ | $ | ||||||
Depreciation expense was $
NOTE 5 – INVESTMENTS
The Company invests a portion of its excess cash balances in short-term debt securities. Investments at August 31, 2021, consisted of corporate bonds with maturities remaining of less than 12 months. The Company may also invest excess cash balances in certificates of deposits, money market accounts, government-sponsored enterprise securities, corporate bonds and/or commercial paper. The Company accounts for its investments in accordance with FASB ASC 320, Investments – Debt and Equity Securities. As of August 31, 2021, all investments were classified as held-to-maturity securities.
The following tables summarize the Company’s short-term investments as of August 31, 2021, and 2020:
| August 31, 2021 | ||||||||||||||||
| (in thousands) | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | ||||||||||||
| Commercial notes (due within one year) | $ | $ | $ | ( | ) | $ | ||||||||||
| Total | $ | $ | $ | ( | ) | $ | ||||||||||
| August 31, 2020 | ||||||||||||||||
| (in thousands) | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | ||||||||||||
| Commercial notes (due within one year) | $ | $ | $ | ( | ) | $ | ||||||||||
| Total | $ | $ | $ | ( | ) | $ | ||||||||||
| F-21 |
NOTE 6 – CONTRACTS PAYABLE
DILIsym Acquisition Liabilities:
On June 1, 2017, we acquired DILIsym. The agreement provided for a working capital adjustment, an eighteen-month $1.0 million holdback provision against certain representations and warranties, and an earnout agreement of up to an additional $5.0 million in earnout payments based on earnings over three years following acquisition. The earnout liability has been recorded at an estimated fair value. Payments under the earnout liability started in fiscal year 2019. In September 2018, $1.6 million was paid out under the first earnout payment, a second earnout payment was made in August 2019 in the amount of $1.7 million. The final payment of $1.8 million was paid in August 2020. In addition, no claims were made against the holdback and the $1.0 million holdback provision was released eighteen months after June 1, 2017.
Lixoft Acquisition Liabilities:
On April 1, 2020, the Company acquired Lixoft. The agreement provided for a 24-month $2.0 million holdback provision against certain representations and warrantees, comprised of $1.3 million of cash and shares of stock valued at $666 thousand issued at the date of the Agreement. In addition, based on a revenue growth formula for the two years subsequent to April 1, 2020, the agreement calls for earnout payments up to $5.5 million (two thirds cash and one-third newly issued, unregistered shares of the Company’s common stock). The former shareholders can earn up to $2.0 million the first year and $3.5 million in year two. In June 2021, $2.0 million was paid to former Lixoft shareholder under the first earnout payment, which was comprised of $1.3 million of cash and $666 thousand worth of common stock.
As of August 31, 2021, and 2020 the following liabilities have been recorded:
| (in thousands) | August 31, 2021 | August 31, 2020 | ||||||
| Holdback Liability | $ | $ | ||||||
| Earnout Liability | ||||||||
| Subtotal | $ | $ | ||||||
| Less: Current Portion | ||||||||
| Long-Term | $ | $ | ||||||
NOTE 7 – COMMITMENTS AND CONTINGENCIES
Leases
Our corporate headquarters is located in Lancaster, California, where we lease 9,255 square feet of office space. The term of the lease extends to January 31, 2026 and the base rent is approximately $17 thousand per month. The lease agreement gives the Company the right, upon 180 days’ prior notice, to opt out of all or part of the last four years of the term, with no penalty.
We lease 12,623 square feet of office space in Buffalo, New York. The initial five-year lease term expired in October 2018; and was renewed for a three-year option, extending it to November 2021 at a base rent of approximately $16 thousand per month. On August 3, 2021, a new lease agreement was signed for a different property for a five-year term at a base rent of approximately $7 thousand per month with an annual 2% increase, and with two, five-year renewal options. Due to ongoing construction, the Company has not yet moved into the new property but anticipates doing so and commencing the lease term no later than November 2021.
We lease approximately 2,700 square feet of space in Research Triangle Park, North Carolina. The initial three-year term was due to expire in October 2020. An amendment to the initial lease became effective on April 1, 2020, which added 686 square feet and extended the term of the lease to September 30, 2023. The new base rent is approximately $8 thousand per month with an annual 3% increase.
| F-22 |
We lease approximately 2,300 square feet of office space in Paris, France. As of April 1, 2020, the lease agreement had minimum payments equaling approximately $288 thousand. The lease is for a 9-year term, with an option to terminate every 3 years, and expires in November of 2024. The base rent is $16 thousand per quarter (approximately $5.3 thousand per month) and can be adjusted each December based on a consumer price index.
Rent expense, including common area maintenance
fees for the years ended August 31, 2021, 2020 and 2019 was $
Lease liability maturities as of August 31, 2021, were as follows:
| (in thousands) Years Ending August 31, | Amount | |||
| 2022 | $ | |||
| 2023 | ||||
| 2024 | ||||
| 2025 | ||||
| 2026 | ||||
| Total undiscounted liabilities | ||||
| Less: imputed interest | ( | ) | ||
| Total future minimum lease payments | $ | |||
Line of Credit
On March 31, 2020, the Company entered into a
Credit Agreement with Wells Fargo Bank, N.A. The Credit Agreement, has provided the Company with a credit facility of $
Employment Agreements
In the normal course of business, the Company has entered into employment agreements with certain of its key management personnel that may require compensation payments upon termination.
License Agreement
The Company had a royalty agreement with Dassault
Systèmes Americas Corp. for access to their Metabolite Database for developing our Metabolite Module within ADMET Predictor. The
module was renamed the Metabolism Module when we released ADMET Predictor version 6 on April 19, 2012. Under this agreement, we paid a
royalty of 25% of revenue derived from the sale of the Metabolism/Metabolite module. This agreement was renegotiated, and the
Company does not bear any royalty obligations towards Dassault Systèmes Americas Corp. effective June 30, 2019. In addition,
the license agreement terminated on September 5, 2020. Under this agreement for the years ended August 31, 2021, 2020 and 2019 we incurred
royalty expense (benefit) of $
Litigation
We are not a party to any legal proceedings and are not aware of any pending legal proceedings of any kind.
| F-23 |
NOTE 8 – SHAREHOLDERS' EQUITY
Shares Outstanding
Shares of common stock outstanding for the years ended August 31, 2021, 2020 and 2019 were as follows:
| August 31, | ||||||||||||
| 2021 | 2020 | 2019 | ||||||||||
| Common stock outstanding, beginning of year | ||||||||||||
| Common stock issued during the year | ||||||||||||
| Common stock outstanding, end of year | ||||||||||||
Dividends
The Company’s Board of Directors declared cash dividends during the years ended August 31, 2021, and 2020. The details of dividends paid are in the following tables:
| (in thousands, except dividend per share) Fiscal Year 2021 | ||||||||||||||
| Record Date | Distribution Date | Number of Shares Outstanding on Record Date | Dividend per Share | Total Amount | ||||||||||
| $ | $ | |||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| Total | $ | |||||||||||||
(in thousands, except dividend per share) Fiscal Year 2020
| Record Date | Distribution Date | Number of Shares Outstanding on Record Date | Dividend per Share | Total Amount | ||||||||||
| $ | $ | |||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| Total | $ | |||||||||||||
| F-24 |
Stock Option Plans
On February 23, 2007, the Board of Directors adopted, and the shareholders approved, the 2007 Stock Option Plan under which a total of shares of common stock had been reserved for issuance. On February 25, 2014 the shareholders approved an additional 1,000,000 shares increasing the total number of shares that may be granted under the Option Plan to 2,000,000. This plan terminated in February 2017 by its term.
On December 23, 2016 the Board of Directors adopted, and on February 23, 2017 the shareholders approved, the 2017 Equity Incentive Plan under which a total of shares of common stock has been reserved for issuance. This plan will terminate in December 2026.
Effective April 9, 2021, the Board of Directors approved, subject to shareholder approval, the adoption of a new 2021 Equity Incentive Plan (the “2021 Plan”) under which million shares are reserved for issuance. The 2021 Plan, which was submitted for shareholder approval at our 2021 Special Meeting of Shareholders held on June 23, 2021, was approved by the shareholders. As a result, the 2021 Plan became effective as of April 9, 2021, and the Company may issue equity awards to permitted recipients thereunder. The maximum contractual life of the plan is ten years.
As of August 31, 2021, employees and directors held Qualified Incentive Stock Options (ISOs) and Non-Qualified Stock Options (“NQSOs”) to purchase 1.2 million shares of common stock at exercise prices ranging from $6.85 to $66.14 per share.
The following tables summarize information about stock options:
| (in thousands,
except per share and weighted-average amounts) Transactions During Fiscal Year 2021 | Number of Options | Weighted-Average Exercise Price Per Share | Weighted-Average Remaining Contractual Life | |||||||||
| Outstanding, August 31, 2020 | $ | |||||||||||
| Granted | ||||||||||||
| Exercised | ( | ) | ||||||||||
| Canceled/Forfeited | ( | ) | ||||||||||
| Outstanding, August 31, 2021 | $ | |||||||||||
| Vested and Exercisable, August 31, 2021 | $ | |||||||||||
| Vested and Expected to Vest, August 31, 2021 | $ | |||||||||||
| (in thousands,
except per share and weighted-average amounts) Transactions During Fiscal Year 2020 | Number of Options | Weighted-Average Exercise Price Per Share | Weighted-Average Remaining Contractual Life | |||||||||
| Outstanding, August 31, 2019 | $ | |||||||||||
| Granted | ||||||||||||
| Exercised | ( | ) | ||||||||||
| Canceled/Forfeited | ( | ) | ||||||||||
| Outstanding, August 31, 2020 | $ | |||||||||||
| Vested and Exercisable, August 31, 2020 | $ | |||||||||||
| Vested and Expected to Vest, August 31, 2020 | $ | |||||||||||
| F-25 |
| (in thousands,
except per share and weighted-average amounts) Transactions During Fiscal Year 2019 | Number of Options | Weighted-Average Exercise Price Per Share | Weighted-Average Remaining Contractual Life | |||||||||
| Outstanding, August 31, 2018 | $ | |||||||||||
| Granted | ||||||||||||
| Exercised | ( | ) | ||||||||||
| Canceled/Forfeited | ( | ) | ||||||||||
| Outstanding, August 31, 2019 | $ | |||||||||||
| Vested and Exercisable, August 31, 2019 | $ | |||||||||||
| Vested and Expected to Vest, August 31, 2019 | $ | |||||||||||
The following table summarizes the Intrinsic Value of options outstanding and options exercisable:
| (in thousands) | Intrinsic Value of Options Outstanding | Intrinsic Value of Options Exercisable | Intrinsic Value of Options Exercised | |||||||||
| Fiscal Year 2019 | $ | $ | $ | |||||||||
| Fiscal Year 2020 | $ | $ | $ | |||||||||
| Fiscal Year 2021 | $ | $ | $ | |||||||||
The weighted-average remaining contractual life of options outstanding issued under the Plans, for both ISOs and NQSOs, was years at August 31, 2021. The total fair value of non-vested stock options as of August 31, 2021, was $ million and is amortizable over a weighted average period of years.
The fair value of these options was estimated at the date of grant using the Black-Scholes option-pricing model. The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options, which do not have vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions, including the expected stock price volatility.
The following table summarizes the fair value of the options, including both ISOs and NQSOs, granted during the current fiscal year 2021 and fiscal year 2020:
| (in thousands, except prices) | Fiscal Year 2021 | Fiscal Year 2020 | ||||||
| Estimated fair value of awards granted | $ | $ | ||||||
| Unvested Forfeiture Rate | % | % | ||||||
| Weighted average grant price | $ | $ | ||||||
| Weighted average market price | $ | $ | ||||||
| Weighted average volatility | % | % | ||||||
| Weighted average risk-free rate | % | % | ||||||
| Weighted average dividend yield | % | % | ||||||
| Weighted average expected life | years | years | ||||||
| F-26 |
The exercise prices for the options outstanding at August 31, 2021, ranged from $6.85 to $66.14, and the information relating to these options is as follows:
(in thousands except prices)
| Exercise Price | Awards Outstanding | Awards Exercisable | ||||||||||||||||||||||||||||
| Low | High | Quantity | Weighted Average Remaining Contractual Life | Weighted Average Exercise Price | Quantity | Weighted Average Remaining Contractual Life | Weighted Average Exercise Price | |||||||||||||||||||||||
| $ | $ | years | $ | years | $ | |||||||||||||||||||||||||
| $ | $ | years | $ | years | $ | |||||||||||||||||||||||||
| $ | $ | years | $ | years | $ | |||||||||||||||||||||||||
| $ | $ | years | $ | years | $ | |||||||||||||||||||||||||
| $ | $ | years | $ | years | $ | |||||||||||||||||||||||||
| $ | $ | years | $ | years | $ | |||||||||||||||||||||||||
| years | $ | years | $ | |||||||||||||||||||||||||||
During the fiscal years ended August 31, 2021, 2020, and 2019, we issued and shares of stock valued at $thousand, $thousand, and $thousand, respectively, to our non-management directors as compensation for board-related duties.
The balance of our par value common stock and
additional paid-in capital as of August 31, 2021, was $
NOTE 9 – INCOME TAXES
We utilize FASB ASC 740-10, “Income Taxes” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns.
Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each year end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. The provision for income taxes represents the tax payable for the period and the change during the period in deferred tax assets and liabilities.
| F-27 |
The components of the income tax provision for the years ended August 31, 2021, 2020 and 2019 were as follows:
| (in thousands) | 2021 | 2020 | 2019 | |||||||||
| Current | ||||||||||||
| Federal | $ | $ | $ | |||||||||
| State | ||||||||||||
| Foreign | ||||||||||||
| Total current tax expense | ||||||||||||
| Deferred | ||||||||||||
| Federal | ( | ) | ( | ) | ( | ) | ||||||
| State | ( | ) | ( | ) | ( | ) | ||||||
| Total deferred federal and state | ( | ) | ( | ) | ( | ) | ||||||
| Total | $ | $ | $ | |||||||||
A reconciliation of the expected income tax computed using the federal statutory income tax rate to the Company's effective income tax rate is as follows for the years ended August 31, 2021, 2020 and 2019:
| 2021 | 2020 | 2019 | ||||||||||
| Income tax computed at federal statutory tax rate | % | % | % | |||||||||
| State taxes, net of federal benefit | ||||||||||||
| Meals & entertainment | ||||||||||||
| Stock based compensation | ( | ) | ( | ) | ( | ) | ||||||
| Other permanent differences | ( | ) | ( | ) | ( | ) | ||||||
| Research and development credit | ( | ) | ( | ) | ( | ) | ||||||
| Foreign tax related differences | ( | ) | ( | ) | ||||||||
| Research & credit adjustments to expense | ||||||||||||
| Change in prior year estimated taxes | ( | ) | ( | ) | ( | ) | ||||||
| Total | % | % | % | |||||||||
| F-28 |
Significant components of the Company's deferred tax assets and liabilities for income taxes for the years ended August 31, 2021, and 2020 are as follows:
| (in thousands) | 2021 | 2020 | ||||||
| Deferred tax assets: | ||||||||
| Accrued payroll and other expenses | $ | $ | ||||||
| Deferred revenue | ||||||||
| Capitalized merger costs | ||||||||
| Intellectual property | ||||||||
| Research and development credits | ||||||||
| State taxes | ||||||||
| Allowance for doubtful accounts | ||||||||
| State tax deferred | ||||||||
| Total deferred tax assets | ||||||||
| Less: Valuation allowance | ||||||||
| Deferred tax asset | ||||||||
| Deferred tax liabilities: | ||||||||
| Property and equipment | ( | ) | ( | ) | ||||
| State tax deferred | ( | ) | ( | ) | ||||
| Intellectual property | ( | ) | ( | ) | ||||
| Capitalized computer software development costs | ( | ) | ( | ) | ||||
| Total deferred tax liabilities | ( | ) | ( | ) | ||||
| Net deferred tax liabilities | $ | ( | ) | $ | ( | ) | ||
We follow guidance issued by the FASB with regard to our accounting for uncertainty in income taxes recognized in the financial statements. Such guidance prescribes a recognition threshold of more likely than not and a measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. In making this assessment, a company must determine whether it is more likely than not that a tax position will be sustained upon examination, based solely on the technical merits of the position and must assume that the tax position will be examined by taxing authorities. Our policy is to include interest and penalties related to income tax expense. Interest and penalties were immaterial for fiscal years 2021, 2020, and 2019, respectively. We file income tax returns with the IRS and various state jurisdictions as well as with the countries of India and France. Our federal income tax returns for fiscal year 2018 thru 2020 are open for audit, and our state tax returns for fiscal year 2017 through 2020 remain open for audit.
Our review of prior year tax positions using the criteria and provisions presented in guidance issued by FASB did not result in a material impact on our financial position or results of operations.
NOTE 10 – CONCENTRATIONS AND UNCERTAINTIES
Financial instruments that potentially subject the Company to concentration of credit risk consist principally of cash, cash equivalents, trade accounts receivable and short-term investments. The Company holds cash and cash equivalents at banks located in California, with balances that often exceed FDIC insured limits. In addition, we hold cash at a bank in France that is not FDIC-insured. Historically, the Company has not experienced any losses in such accounts. However, we are investigating alternative way to minimize our exposure to such risk. While the Company may be exposed to credit losses due to the nonperformance of its counterparties, the Company does not expect the settlement of these transactions to have a material effect on its results of operations, cash flows or financial condition. The Company maintains cash at financial institutions that may, at times, exceed federally insured limits.
| F-29 |
Revenue concentration shows that
international sales accounted for
Accounts receivable concentrations show that three
customers each comprised between
We operate in the computer software industry, which is highly competitive and changes rapidly. Our operating results could be significantly affected by our ability to develop new products and find new distribution channels for new and existing products.
The majority of our customers are in the pharmaceutical industry. During economic downturns, we have seen consolidations in the pharmaceutical industry. The extent to which the COVID-19 pandemic impacts our business going forward will depend on numerous factors we cannot reliably predict, including the duration and scope of the pandemic; businesses and individuals' actions in response to the pandemic; and the impact on economic activity including the possibility of recession or financial market instability. These factors may adversely impact consumer, business, and government spending as well as customers' ability to pay for our products and services on an ongoing basis. As a result, our growth rate could be affected by consolidation and downsizing in the pharmaceutical industry.
NOTE 11 – SEGMENT AND GEOGRAPHIC REPORTING
We account for segments and geographic revenues in accordance with guidance issued by the FASB. Our reportable segments are strategic business units that offer different products and services.
Results for each divisional segment and consolidated results are as follows for the years ended August 31, 2021, 2020 and 2019:
| (in thousands) | Year ended August 31, 2021 | |||||||||||||||||||||||
| Simulations Plus | Cognigen | DILIsym | Lixoft | Eliminations | Total | |||||||||||||||||||
| Revenues | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Income (loss) from operations | $ | $ | $ | ( | ) | $ | $ | $ | ||||||||||||||||
| Total assets | $ | $ | $ | $ | $ | ( | ) | $ | ||||||||||||||||
| Goodwill | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Capital expenditures | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Capitalized software costs | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Depreciation and amortization | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| (in thousands) | Year ended August 31, 2020 | |||||||||||||||||||||||
| Simulations Plus | Cognigen | DILIsym | Lixoft* | Eliminations | Total | |||||||||||||||||||
| Revenues | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Income from operations | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Total assets | $ | $ | $ | $ | $ | ( | ) | $ | ||||||||||||||||
| Goodwill | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Capital expenditures | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Capitalized software costs | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Depreciation and amortization | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| * |
| F-30 |
| (in thousands) | Year ended August 31, 2019 | |||||||||||||||||||
| Simulations Plus | Cognigen | DILIsym | Eliminations | Total | ||||||||||||||||
| Revenues | $ | $ | $ | $ | $ | |||||||||||||||
| Income from operations | $ | $ | $ | $ | $ | |||||||||||||||
| Total assets | $ | $ | $ | $ | ( | ) | $ | |||||||||||||
| Goodwill | $ | $ | $ | $ | $ | |||||||||||||||
| Capital expenditures | $ | $ | $ | $ | $ | |||||||||||||||
| Capitalized software costs | $ | $ | $ | $ | $ | |||||||||||||||
| Depreciation and amortization | $ | $ | $ | $ | $ | |||||||||||||||
Results for each business unit segment and consolidated results for the years ended August 31, 2021, 2020 and 2019 were as follows:
| (in thousands) | Year ended August 31, 2021 | |||||||||||
| Software | Services | Total | ||||||||||
| Revenues | $ | $ | $ | |||||||||
| Cost of revenues | ||||||||||||
| Gross profit | $ | $ | $ | |||||||||
| Gross margin | ||||||||||||
Our software business and services business represented 60% and 40% of total revenue, respectively, for the year ended August 31, 2021.
| (in thousands) | Year ended August 31, 2020 | |||||||||||
| Software | Services | Total | ||||||||||
| Revenues | $ | $ | $ | |||||||||
| Cost of revenues | ||||||||||||
| Gross profit | $ | $ | $ | |||||||||
| Gross margin | ||||||||||||
Our software business and services business represented 52% and 48% of total revenue, respectively, for the 2020 fiscal year.
| (in thousands) | Year ended August 31, 2019 | |||||||||||
| Software | Services | Total | ||||||||||
| Revenues | $ | $ | $ | |||||||||
| Cost of revenues | ||||||||||||
| Gross profit | $ | $ | $ | |||||||||
| Gross margin | ||||||||||||
Our software business and services business represented 54% and 46% of total revenue, respectively, for the 2019 fiscal year.
| F-31 |
In addition, the Company allocates revenues to geographic areas based on the locations of its customers. Geographical revenues for the years ended August 31, 2021, 2020 and 2019 were as follows:
| (in thousands) | Year ended August 31, | |||||||||||||||||||||||
| 2021 | 2020 | 2019 | ||||||||||||||||||||||
| $ | % of total | $ | % of total | $ | % of total | |||||||||||||||||||
| Americas | $ | % | $ | % | $ | % | ||||||||||||||||||
| EMEA | ||||||||||||||||||||||||
| Asia Pacific | ||||||||||||||||||||||||
| Total | $ | % | $ | % | $ | % | ||||||||||||||||||
NOTE 12 – RELATED PARTY TRANSACTIONS
On April 1, 2020, the Company acquired Lixoft.
As part of that agreement the Company paid $ million and issued stock with a value of $ million to former shareholders of Lixoft,
some who are currently employees of the Company. In addition, as part of the acquisition agreement the Company owes approximately $
thousand of acquisition liabilities at August 31, 2021, to the former shareholders who are still employees of the Company. During the
fiscal year 2021, under the terms of the agreement, the Company made payments totaling $
NOTE 13 – EMPLOYEE BENEFIT PLAN
We maintain a 401(k) Plan for eligible employees.
We make matching contributions equal to 100% of the employee’s elective deferral, not to exceed 4% of the total employee compensation.
We can also elect to make a profit-sharing contribution. We contributed $
NOTE 14 – ACQUISITION
On March 31, 2020, the Company entered into a Stock Purchase and Contribution Agreement (the “Agreement”) with Lixoft, a French société par actions simplifiée (“Lixoft”). On April 1, 2020, the Company consummated the acquisition of all outstanding equity interests of Lixoft pursuant to the terms of the Agreement, with Lixoft becoming a wholly-owned subsidiary of the Company. We believe the combination of Simulations Plus and Lixoft provides substantial future potential based on the complementary strengths of each of the companies.
Under the terms of the Agreement, as described below, the Company will pay the former shareholders of Lixoft total consideration of up to $16.5 million, consisting of two-thirds cash and one-third newly issued, unregistered shares of the Company’s common stock. In addition, the Company will pay $3,456,029 of excess working capital based on the March 31, 2020 financial statements of Lixoft.
On April 1, 2020, the Company paid the former shareholders of Lixoft a total of $10.8 million, comprised of cash in the amount of $9.5 million and the issuance of 111,682 shares of the Company’s common stock valued at $3.7 million, net of adjustments and a holdback for representations and warranties (under the terms of the Agreement a price of approximately $32.15 dollars per share was used based upon the volume-weighted average closing price of the Company’s shares of common stock for the 30-consecutive-trading-day period ending two trading days prior to April 1, 2020). 9,669 shares are held in an escrow for offset for representations and warrantees. Within three business days following the two-year anniversary of March 31, 2020 (the date of the Agreement) and subject to any offsets for representations and warrantees, the Company will pay the former shareholders of Lixoft a total of $2.0 million, comprised of $1.3 million of cash and the release from an escrow shares of stock valued at $666 thousand issued at the date of the Agreement. The Agreement provides for a two-year market standoff period in which the newly issued shares may not be sold by the recipients thereof.
| F-32 |
In addition, the agreement calls for earnout payments up to an additional $5.5 million, two-thirds cash and one-third newly issued, unregistered shares of the Company’s common stock based on a revenue growth formula each year for the two years subsequent to April 1, 2020. The former shareholders can earn up to $2.0 million the first year and $3.5 million in year two. The earnout liability has been recorded at fair value.
Under the acquisition method of accounting, the total purchase price reflects Lixoft’s tangible and intangible assets and liabilities based on their estimated fair values at the date of the completion of the acquisition (April 1, 2020). The following table summarizes the preliminary allocation of the purchase price for Lixoft:
| (in thousands) | ||||
| Assets acquired, including cash of $3,799 and accounts receivable of $629 | $ | |||
| Developed technologies acquired | ||||
| Estimated value of intangible assets acquired (customer lists, trade name etc.) | ||||
| Estimated goodwill acquired | ||||
| Liabilities assumed | ( | ) | ||
| Total consideration | $ | |||
Goodwill has been provided in the transaction based on estimates of future earnings of this subsidiary including anticipated synergies associated with the positioning of the combined company as a leader in model-based drug development.
Consolidated Supplemental Pro Forma Information
The following unaudited consolidated supplemental pro forma information assumes that the acquisition of Lixoft took place on September 1, 2017 for the income statement years ended August 31, 2020. These amounts have been calculated after applying the Company’s accounting policies and adjusting the results of Lixoft to reflect the same expenses in the years ended August 31, 2019 and 2018. The adjustments include costs of acquisition, and amortization of intangibles and other technologies acquired during the merger, assuming the fair-value adjustments applied on September 1, 2017, together with consequential tax effects.
| (Actual) | (Pro forma) | (Pro forma) | ||||||||||
| 2021 | 2020* | 2019 | ||||||||||
| (in thousands) | (Audited) | (unaudited) | (unaudited) | |||||||||
| Revenue | $ | $ | $ | |||||||||
| Net Income | $ | $ | $ | |||||||||
| * |
| F-33 |
NOTE 15 – UNAUDITED QUARTERLY FINANCIAL DATA
The following table presents selected unaudited quarterly financial data for each full quarterly period for the years ended August 31, 2021, and 2020:
| (in thousands) | Year ended August 31, 2021 | |||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| Quarter | Quarter | Quarter | Quarter | |||||||||||||
| Revenues | $ | $ | $ | $ | ||||||||||||
| Gross profit | $ | $ | $ | $ | ||||||||||||
| Net income | $ | $ | $ | $ | ||||||||||||
| Earnings per share, basic | $ | $ | $ | $ | ||||||||||||
| Earnings per share, diluted | $ | $ | $ | $ | ||||||||||||
| (in thousands) | Year ended August 31, 2020 | |||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| Quarter | Quarter | Quarter | Quarter | |||||||||||||
| Revenues | $ | $ | $ | $ | ||||||||||||
| Gross profit | $ | $ | $ | $ | ||||||||||||
| Net income | $ | $ | $ | $ | ||||||||||||
| Earnings per share, basic | $ | $ | $ | $ | ||||||||||||
| Earnings per share, diluted | $ | $ | $ | $ | ||||||||||||
NOTE 16 - SUBSEQUENT EVENTS
Dividend Declared
On Wednesday, October 13, 2021, our Board of Directors declared a quarterly cash dividend of $0.06 per share to our shareholders. The dividend in the amount of $1.2 million will be distributed on Monday, November 1, 2021, for shareholders of record as of Monday, October 25, 2021.
Effective September 1, 2021, the Company merged both Cognigen Corporation and DILIsym, Services, Inc. with and into Simulations Plus, Inc. through short form mergers (the “Mergers”). To effectuate the Mergers, the Company filed Certificates of Ownership with the Secretaries of State of the states of Delaware (Cognigen’s and DILIsym’s state of incorporation) and California (the Company’s state of incorporation). Consummation of the Mergers was not subject to approval of the Company’s stockholders and did not impact the rights of the Company’s stockholders.
| F-34 |