Exhibit 99.1

OPCO Fireside Chat December 17, 2020 Nasdaq: SLP

2 With the exception of historical information, the matters discussed in this presentation are forward - looking statements that involve a number of risks and uncertainties . The actual results of the Company could differ significantly from those statements . Factors that could cause or contribute to such differences include but are not limited to : continuing demand for the Company’s products, competitive factors, the Company’s ability to finance future growth, the Company’s ability to produce and market new products in a timely fashion, the Company’s ability to continue to attract and retain skilled personnel, and the Company’s ability to sustain or improve current levels of productivity . Further information on the Company’s risk factors is contained in the Company’s quarterly and annual reports and filed with the Securities and Exchange Commission . Safe Harbor Statement

Modeling and Simulation in Pharma Drug Development Software: The most comprehensive and widely recognized set of tools for in silico drug development. Ongoing development and reinvestment to incorporate the latest science and ensure a seamless user experience. Services: Highly interactive collaboration with our renowned experts allows us to deliver results in timely fashion and ensures a top quality deliverable. • Regular interactions and frequent progress updates eliminate surprises and ensure relevance as the knowledge - base evolves • Synergies come from shared knowledge between client and consultant • We welcome involvement, participation, and input from stakeholders outside of M&S 3 NASDAQ: SLP
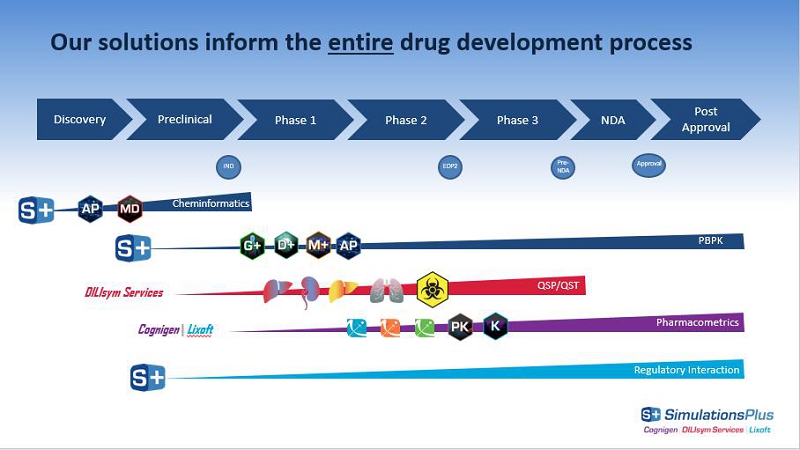
Our solutions inform the entire drug development process Discovery Phase 1 Phase 2 Phase 3 NDA Cheminformatics EOP2 Pre - NDA IND Pharmacometrics PBPK Preclinical Regulatory Interaction Post Approval Approval Cognigen | Lixoft DILIsym Services QSP/QST
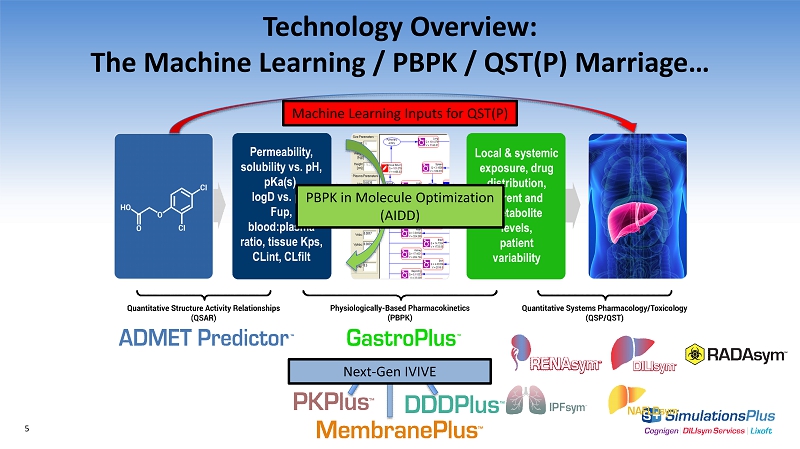
Technology Overview: The Machine Learning / PBPK / QST(P) M arriage… Permeability, solubility vs. pH, pKa(s), logD vs. pH, Fup, blood:plasma ratio, tissue Kps, CLint, C L filt Local & systemic exposure, drug distribution, parent and metabolite levels, patient variability 5 Machine Learning Inputs for QST(P) Next - Gen IVIVE PBPK in Molecule Optimization (AIDD)
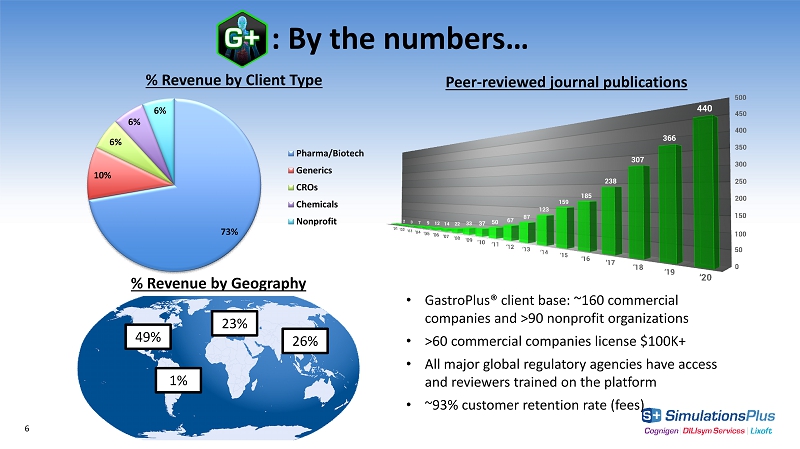
: By the numbers… 6 Peer - reviewed journal publications 73% 10% 6% 6% 6% % Revenue by Client Type Pharma/Biotech Generics CROs Chemicals Nonprofit % Revenue by Geography 49% 1% 23% 26% • GastroPlus® client base: ~160 commercial companies and >90 nonprofit organizations • >60 commercial companies license $100K+ • All major global regulatory agencies have access and reviewers trained on the platform • ~93% customer retention rate (fees)
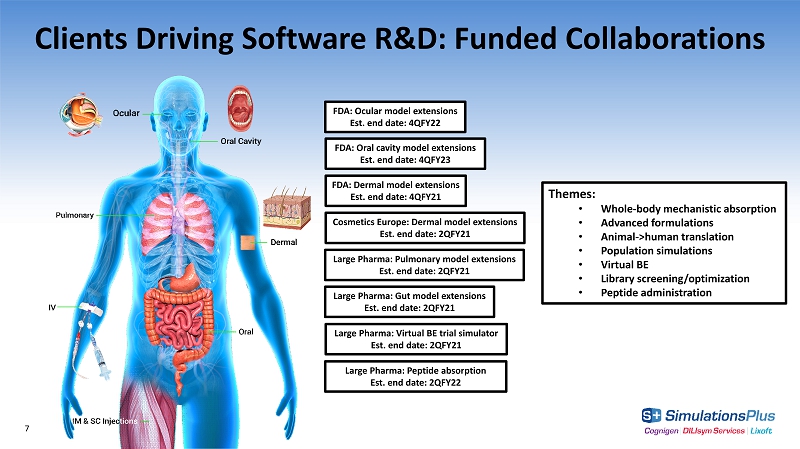
Cosmetics Europe: Dermal model extensions Est. end date: 2QFY21 Clients Driving Software R&D: Funded Collaborations Large Pharma: Virtual BE trial simulator Est. end date: 2QFY21 FDA: Dermal model extensions Est. end date: 4QFY21 7 Themes: • Whole - body mechanistic absorption • Advanced formulations • Animal - >human translation • Population simulations • Virtual BE • Library screening/optimization • Peptide administration Large Pharma: Gut model extensions Est. end date: 2QFY21 FDA: Ocular model extensions Est. end date: 4QFY22 FDA: Oral cavity model extensions Est. end date: 4QFY23 Large Pharma: Peptide absorption Est. end date: 2QFY22 Large Pharma: Pulmonary model extensions Est. end date: 2QFY21

>70 Approved drug product applications supported by GastroPlus® simulations 8
 \
\
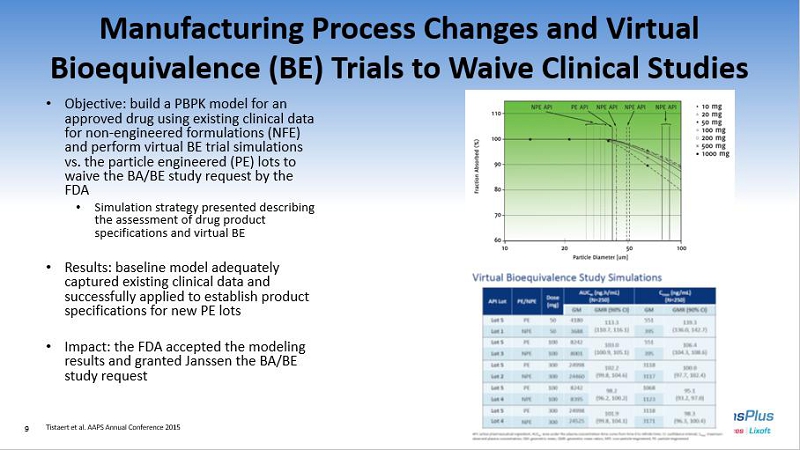
Manufacturing Process Changes and Virtual Bioequivalence (BE) Trials to Waive Clinical Studies • Objective: build a PBPK model for an approved drug using existing clinical data for non - engineered formulations (NFE) and perform virtual BE trial simulations vs. the particle engineered (PE) lots to waive the BA/BE study request by the FDA • Simulation strategy presented describing the assessment of drug product specifications and virtual BE • Results: baseline model adequately captured existing clinical data and successfully applied to establish product specifications for new PE lots • Impact: the FDA accepted the modeling results and granted Janssen the BA/BE study request Tistaert et al. AAPS Annual Conference 2015 9
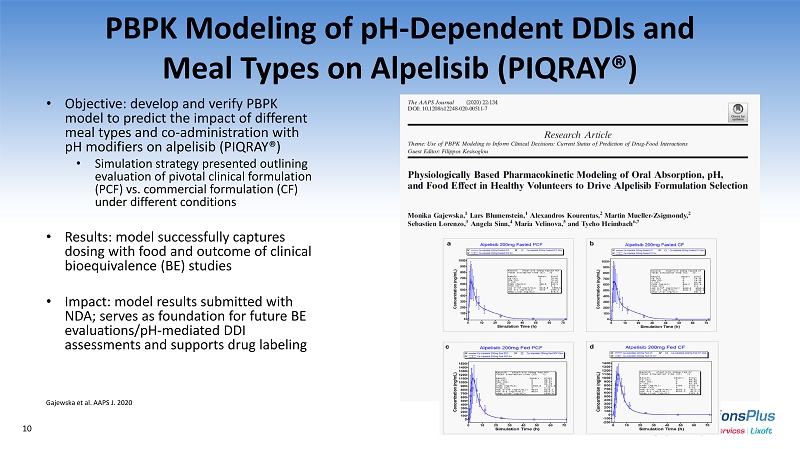
PBPK Modeling of pH - Dependent DDIs and Meal Types on Alpelisib (PIQRAY®) • Objective: develop and verify PBPK model to predict the impact of different meal types and co - administration with pH modifiers on alpelisib (PIQRAY®) • Simulation strategy presented outlining evaluation of pivotal clinical formulation (PCF) vs. commercial formulation (CF) under different conditions • Results: model successfully captures dosing with food and outcome of clinical bioequivalence (BE) studies • Impact: model results submitted with NDA; serves as foundation for future BE evaluations/pH - mediated DDI assessments and supports drug labeling Gajewska et al. AAPS J. 2020 10

Establish Dissolution Safe Space in Adult and Pediatric Populations (TAMIFLU®) 11 • Objective: develop and verify PBPK model to predict the exposure of oseltamivir (TAMIFLU®) and its main metabolite in adult and pediatric populations • Simulation strategy presented model development in adults and extrapolation to pediatrics at different age groups • Results: model successfully captures active and metabolite exposure across population groups and defines dissolution safe spaces unique to each one • Impact: previous model supported dose selection and trial design in pediatrics; optimized model supports future manufacturing site/formulation changes and sets clinically relevant safe spaces in both adults and pediatrics. Miao et al. AAPS J. 2020

DILIsym Services Inc., an SLP Company • DILIsym Services, Inc. offers comprehensive program services: – DILIsym software licensing, training, development (consortia) – NAFLDsym and IPFsym software licensing, training, development – QSP / QST simulation consulting projects – Consulting and data interpretation; in vitro assay experimental design and management – RENAsym and RADAsym software in development “Our vision is safer, effective, more affordable medicines for patients through modeling and simulation.” 12
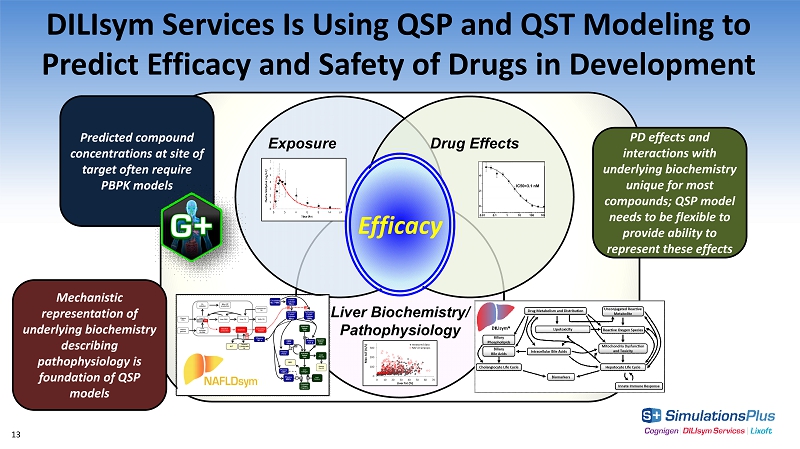
DILIsym Services Is Using QSP and QST Modeling to Predict Efficacy and Safety of Drugs in Development Exposure Drug Effects DILI Liver Biochemistry/ Pathophysiology Efficacy Mechanistic representation of underlying biochemistry describing pathophysiology is foundation of QSP models PD effects and interactions with underlying biochemistry unique for most compounds; QSP model needs to be flexible to provide ability to represent these effects Predicted compound concentrations at site of target often require PBPK models 13
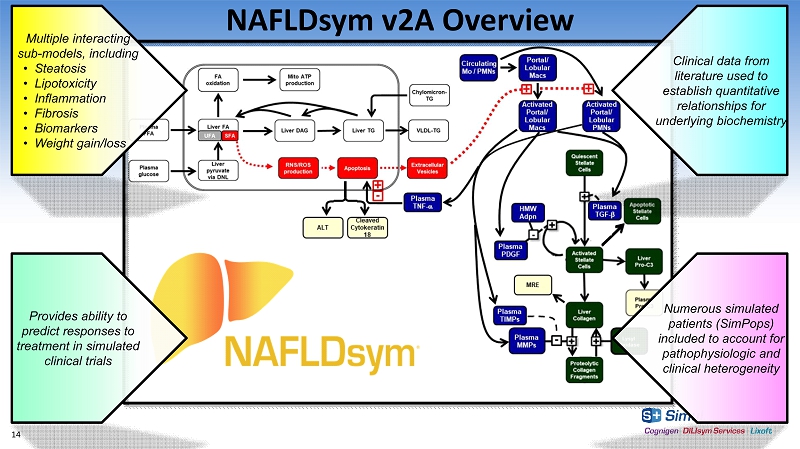
NAFLDsym v2A Overview Multiple interacting sub - models, including • Steatosis • Lipotoxicity • Inflammation • Fibrosis • Biomarkers • Weight gain/loss Numerous simulated patients (SimPops) included to account for pathophysiologic and clinical heterogeneity Clinical data from literature used to establish quantitative relationships for underlying biochemistry Provides ability to predict responses to treatment in simulated clinical trials 14
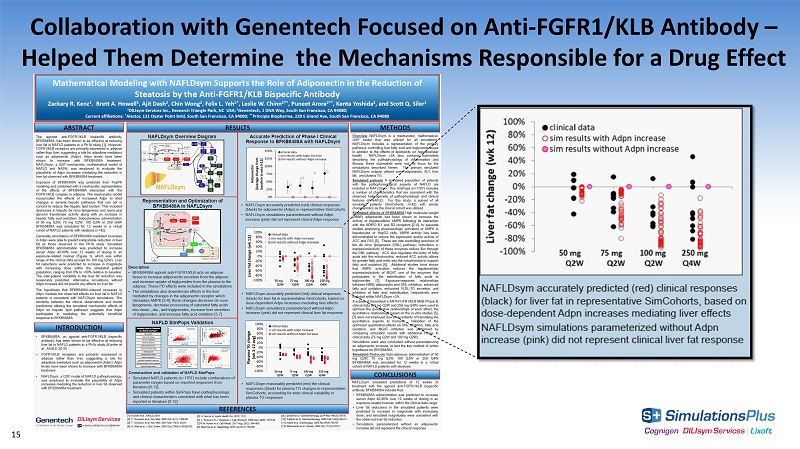
Collaboration with Genentech Focused on Anti - FGFR1/KLB Antibody – Helped Them Determine the Mechanisms Responsible for a Drug Effect 15
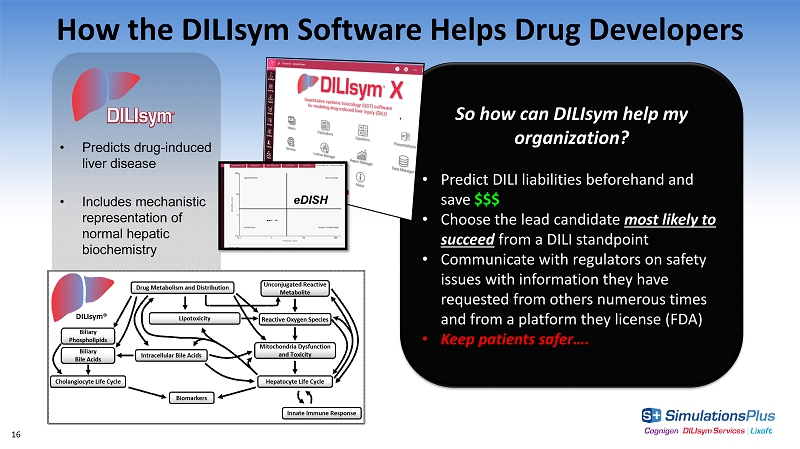
How the DILIsym Software Helps Drug Developers • Predicts drug - induced liver disease • Includes mechanistic representation of normal hepatic biochemistry So how can DILIsym help my organization? • Predict DILI liabilities beforehand and save $$$ • Choose the lead candidate most likely to succeed from a DILI standpoint • Communicate with regulators on safety issues with information they have requested from others numerous times and from a platform they license (FDA) • Keep patients safer…. eDISH 16
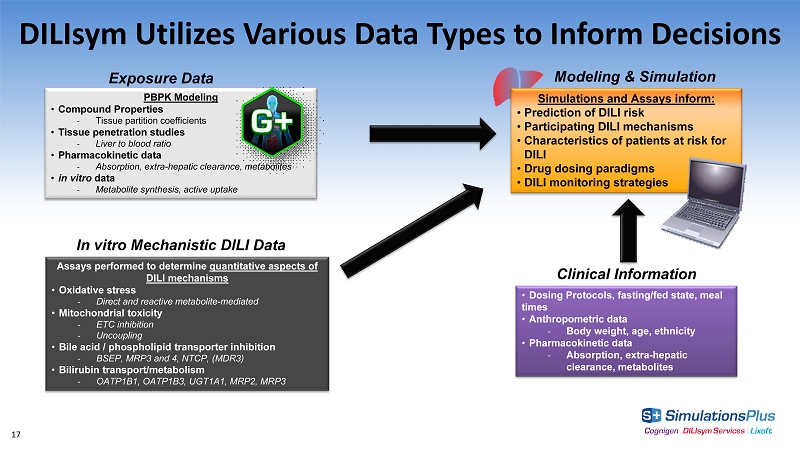
DILIsym Utilizes Various Data Types to Inform Decisions • Dosing Protocols, fasting/fed state, meal times • Anthropometric data - Body weight, age, ethnicity • Pharmacokinetic data - Absorption, extra - hepatic clearance, metabolites PBPK Modeling • Compound Properties - Tissue partition coefficients • Tissue penetration studies - Liver to blood ratio • Pharmacokinetic data - Absorption, extra - hepatic clearance, metabolites • in vitro data - Metabolite synthesis, active uptake Modeling & Simulation In vitro Mechanistic DILI Data Clinical Information Assays performed to determine quantitative aspects of DILI mechanisms • Oxidative stress - Direct and reactive metabolite - mediated • Mitochondrial toxicity - ETC inhibition - Uncoupling • Bile acid / phospholipid transporter inhibition - BSEP, MRP3 and 4, NTCP, (MDR3) • Bilirubin transport/metabolism - OATP1B1, OATP1B3, UGT1A1, MRP2, MRP3 Exposure Data Simulations and Assays inform: • Prediction of DILI risk • Participating DILI mechanisms • Characteristics of patients at risk for DILI • Drug dosing paradigms • DILI monitoring strategies 17

Important DILIsym Application Examples NORMAL 18
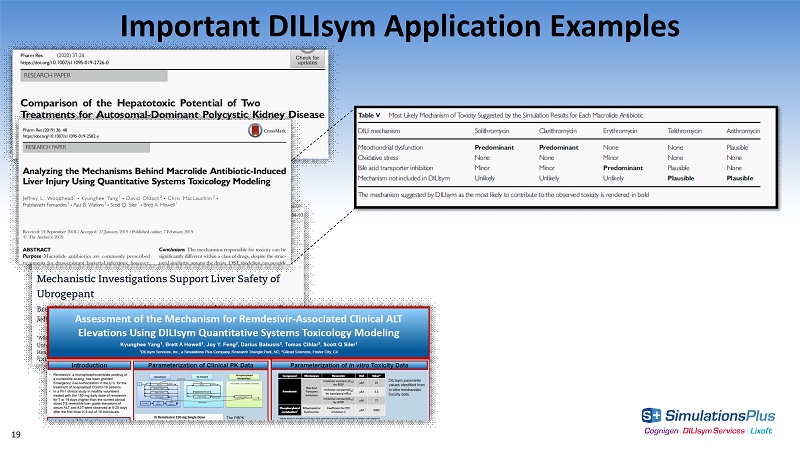
19 Important DILIsym Application Examples

20 Important DILIsym Application Examples

21 Important DILIsym Application Examples

Q & A 22