Exhibit 99.1

| BIO Investor Forum 2008 San Francisco, CA 30 October, 2008 |

| Safe Harbor Statement Statements herein relating to future financial or business performance, conditions or strategies and other financial and business matters, including expectations regarding clinical development, product sales, operating expenses, and anticipated milestones are forward-looking statements within the meaning of the Private Securities Litigation Reform Act. Novavax cautions that these forward-looking statements are subject to numerous assumptions, risks and uncertainties, which change over time. Such factors that may cause actual results to differ materially from the results discussed in the forward-looking statements or historical experience include risks relating to the early stage of Novavax's product candidates under development; current results may not be predictive of future pandemic results, results of our seasonal influenza vaccine or any other vaccine that we may develop; further testing is required before regulatory approval can be applied for and the FDA may not approve a vaccine even if further trial results are similar to those disclosed previously by the company; uncertainties relating to clinical trials, including possible delays in initiating or completing the trials and safety and immunogenicity results; dependence on the efforts of third parties; competition for clinical resources and patient enrollment from drug candidates in development by other companies with greater resources and visibility; and risks that we may lack the financial resources and access to capital to fund our operations including further clinical trials. Further information on the factors and risks that could affect Novavax's business, financial conditions and results of operations, is contained in Novavax's filings with the U.S. Securities and Exchange Commission, which are available at http://www.sec.gov. These forward-looking statements speak only as of the date of this presentation, and Novavax assumes no duty to update forward-looking statements. |

| Creating Tomorrow's Vaccines Robust vaccine pipeline Positive Phase II results with H5N1 Flu VLP (POC); Seasonal Flu VLP in Phase II Competitive advantage e.g., influenza vaccines (COGS) Reduced Capital Requirement Multiple commercialization options Efficient Manufacturing Solution Scalable Cell Based Manufacturing Broad VLP Platform Technology Increased Financing Options Govt., NGOs, Partnering, Equity Financing Flexibility |
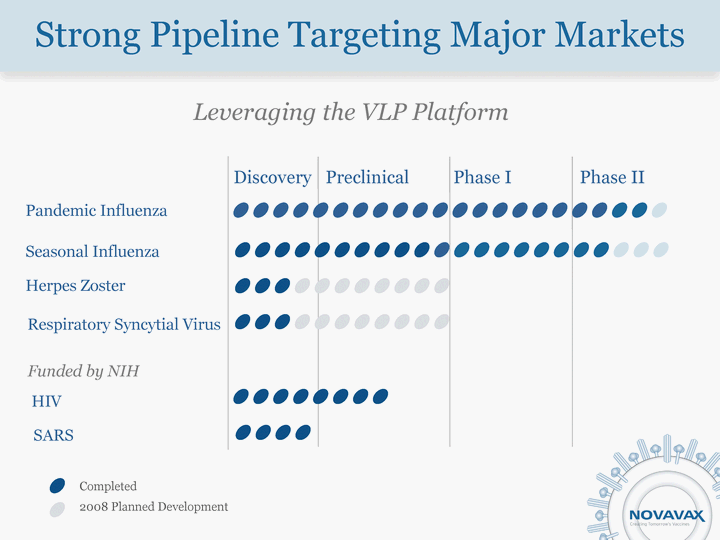
| Completed 2008 Planned Development Leveraging the VLP Platform Discovery Preclinical Phase I Phase II Pandemic Influenza Seasonal Influenza Herpes Zoster Respiratory Syncytial Virus Funded by NIH HIV SARS Strong Pipeline Targeting Major Markets |
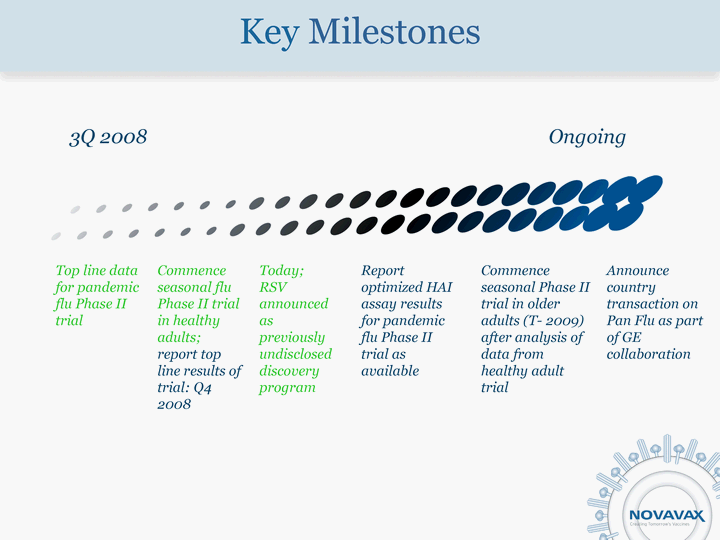
| Key Milestones Top line data for pandemic flu Phase II trial Commence seasonal flu Phase II trial in healthy adults; report top line results of trial: Q4 2008 Today; RSV announced as previously undisclosed discovery program Commence seasonal Phase II trial in older adults (T- 2009) after analysis of data from healthy adult trial Announce country transaction on Pan Flu as part of GE collaboration 3Q 2008 Ongoing Report optimized HAI assay results for pandemic flu Phase II trial as available |
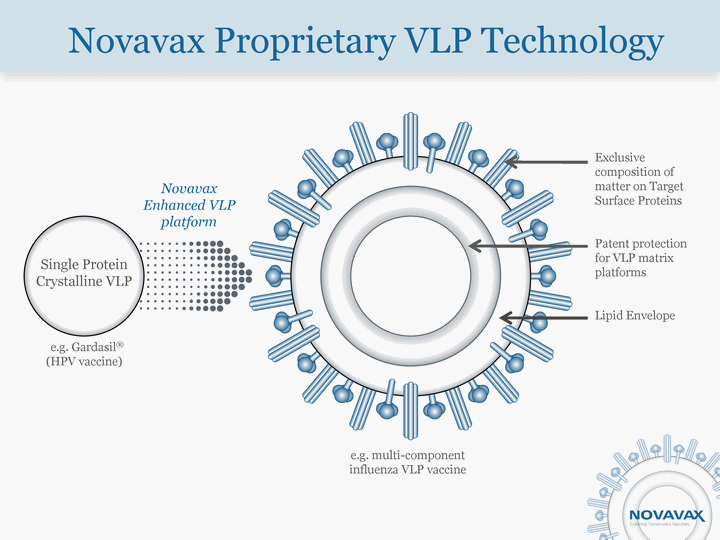
| Novavax Proprietary VLP Technology Single Protein Crystalline VLP e.g. Gardasil(r) (HPV vaccine) Novavax Enhanced VLP platform e.g. multi-component influenza VLP vaccine Exclusive composition of matter on Target Surface Proteins Patent protection for VLP matrix platforms Lipid Envelope |
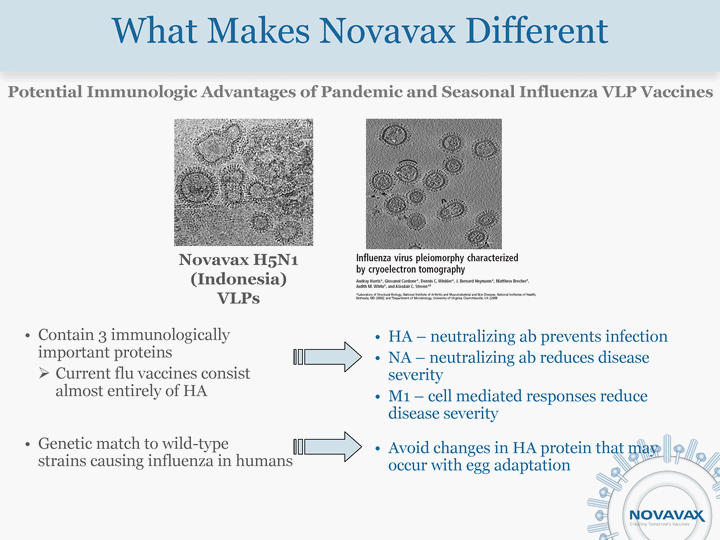
| What Makes Novavax Different Contain 3 immunologically important proteins Current flu vaccines consist almost entirely of HA Genetic match to wild-type strains causing influenza in humans HA - neutralizing ab prevents infection NA - neutralizing ab reduces disease severity M1 - cell mediated responses reduce disease severity Avoid changes in HA protein that may occur with egg adaptation Novavax H5N1 (Indonesia) VLPs Potential Immunologic Advantages of Pandemic and Seasonal Influenza VLP Vaccines |

| Proof of concept for VLP technology Safe, well-tolerated and immunogenic at two doses; 230 subjects Significant rise in antibody titers vs. baseline Support development of VLP vaccines against seasonal influenza and other diseases Pandemic Influenza Vaccine: Positive Phase I/II Results |
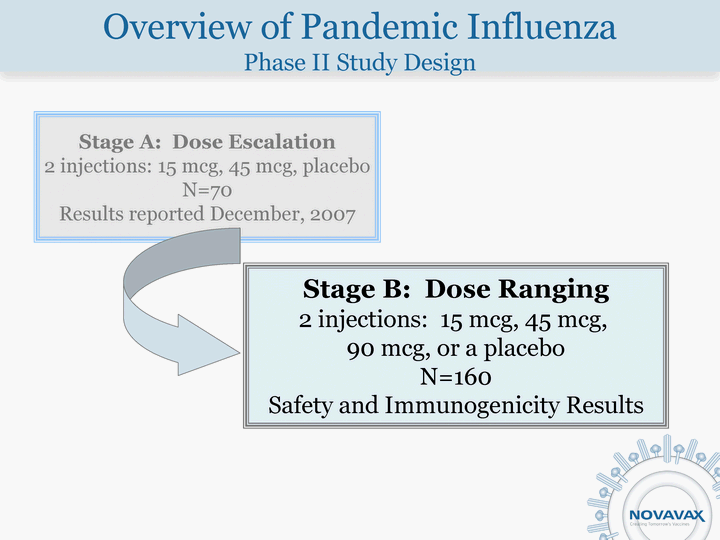
| Overview of Pandemic Influenza Phase II Study Design Stage A: Dose Escalation 2 injections: 15 mcg, 45 mcg, placebo N=70 Results reported December, 2007 Stage B: Dose Ranging 2 injections: 15 mcg, 45 mcg, 90 mcg, or a placebo N=160 Safety and Immunogenicity Results |

| Immunogenicity Percentage of subjects with NA titer >1:20 (>4-fold rise in titer) 15 mcg - 72% (CI: 53,86) 45 mcg - 73% (CI: 55,87) 90 mcg - 94% (CI: 80,99) Placebo - 0% Neutralizing Antibody (NA) Responses Against the A/Indonesia Strain After 2 Doses of the H5N1 VLP Vaccine |

| Stockpile, capacity pre-purchase, Domestic vaccine production capacity Pandemic Influenza Vaccine Opportunity Potential Market Worldwide population at risk Vaccine market segments Insufficient worldwide vaccine capacity Global budgets for pandemic preparedness > $13 billion Human infections/deaths continue Asia particularly vulnerable Supply gap > 6 billion doses Regional or local solution ideal |
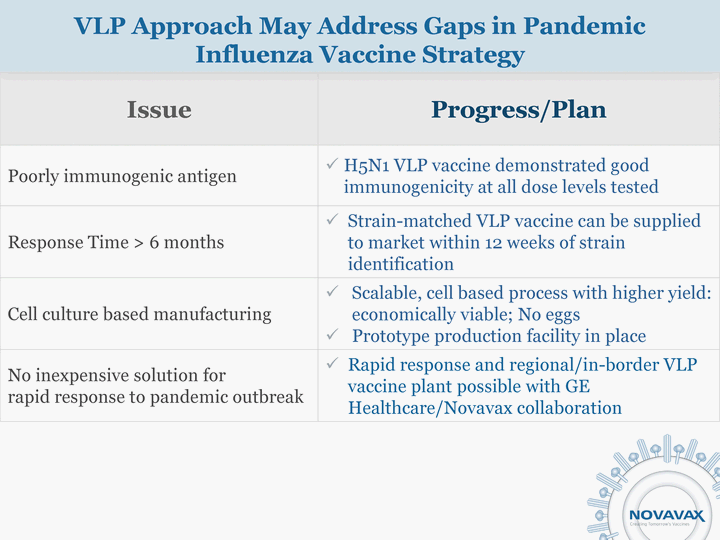
| Issue Progress/Plan Poorly immunogenic antigen H5N1 VLP vaccine demonstrated good immunogenicity at all dose levels tested Response Time > 6 months Strain-matched VLP vaccine can be supplied to market within 12 weeks of strain identification Cell culture based manufacturing Scalable, cell based process with higher yield: economically viable; No eggs Prototype production facility in place No inexpensive solution for rapid response to pandemic outbreak Rapid response and regional/in-border VLP vaccine plant possible with GE Healthcare/Novavax collaboration VLP Approach May Address Gaps in Pandemic Influenza Vaccine Strategy |
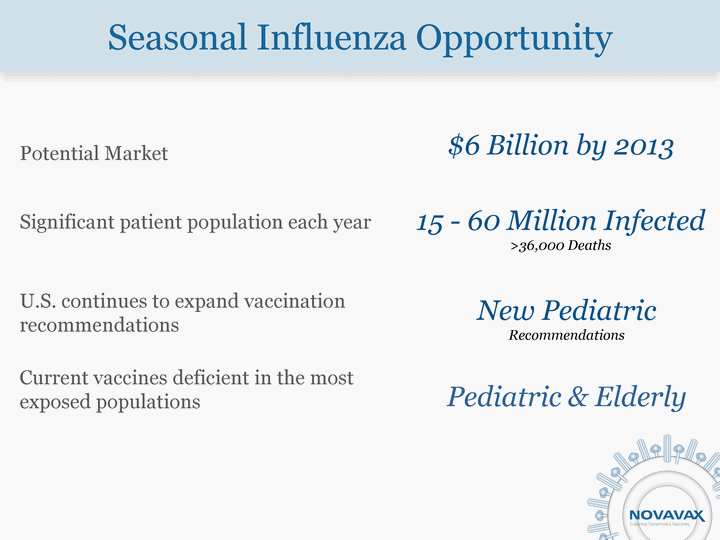
| Seasonal Influenza Opportunity Potential Market Significant patient population each year U.S. continues to expand vaccination recommendations Current vaccines deficient in the most exposed populations $6 Billion by 2013 15 - 60 Million Infected >36,000 Deaths New Pediatric Recommendations Pediatric & Elderly |

| Overview of Seasonal Influenza Phase II Study Designs Dose Ranging in Healthy Adults 1 injection: 5 mcg, 15 mcg, 30 mcg, or a placebo N=~300 No serious adverse events reported to date Blinded data show different safety profile vs. pandemic vaccine Primary results targeted December, 2008 Dose Ranging in Elderly Adults 1 injection; 2-3 different doses Study start delayed to 2009 Study design will be based on results of trial in healthy adults |

| Issue Progress/Plan Over-supply of current vaccine; Limited effectiveness in elderly, pediatrics Probability of success for differentiation increased with H5N1 vaccine data; dose response observed Phase IIa dose ranging study in healthy adults ongoing Phase IIa dose ranging in Older adults to commence in 2009 Strain mismatch based vaccine ineffectiveness Functional Neuraminidase, M1 in VLP vaccine Reliable supply and speed to market with annual strain changes 2008/09 strain seeds created within 6 weeks of CDC announcement Cell culture based manufacturing Scalable, cell based process in place, No eggs 10MM dose facility in place at current yields VLP Approach May Address Gaps in Current Seasonal Influenza Vaccines |

| Seasonal Flu - Window of Opportunity How Does Novavax Compete? Company Differentiation in Elderly Indication for Broad Population (>6 mos) Cell-culture Speed to Market Each Year High dose or intradermal Currently have No No MF59-adj vaccine (not licensed in US) Yes No No Novel Adjuvant (regulatory risk) Yes No No No 2 - 49 yrs No No No Yes No No Protein Sciences No (not licensed) No Yes Yes; Possible Wait for data - sound basis Focus on >=3yrs Yes Probable 2013 WW Seasonal Market >$6.0Bn |

| Herpes Zoster (Shingles) Virus Discovery Candidate Potential multi-billion dollar market: ACIP recommended for ages 60+; approx 50 million patient market potential in US alone Lifetime risk of disease, 25% - 30% Global opportunity Single vaccine on market ($150/dose): Live, stored at -15°C; must be used within 30 minutes; limited supply Preclinical vaccine candidates in testing |
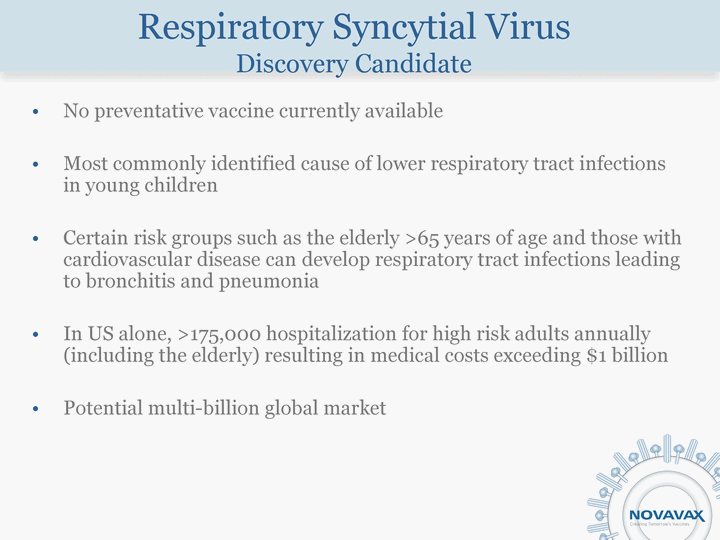
| Respiratory Syncytial Virus Discovery Candidate No preventative vaccine currently available Most commonly identified cause of lower respiratory tract infections in young children Certain risk groups such as the elderly >65 years of age and those with cardiovascular disease can develop respiratory tract infections leading to bronchitis and pneumonia In US alone, >175,000 hospitalization for high risk adults annually (including the elderly) resulting in medical costs exceeding $1 billion Potential multi-billion global market |

| Old Manufacturing Solution |
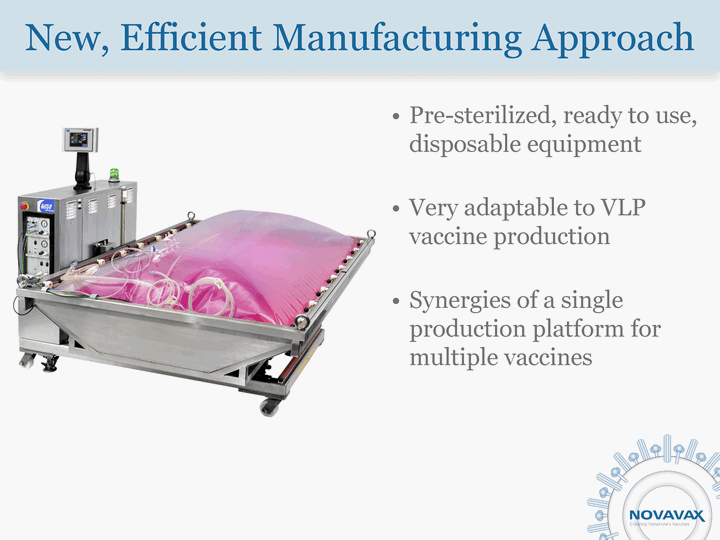
| New, Efficient Manufacturing Approach Pre-sterilized, ready to use, disposable equipment Very adaptable to VLP vaccine production Synergies of a single production platform for multiple vaccines |
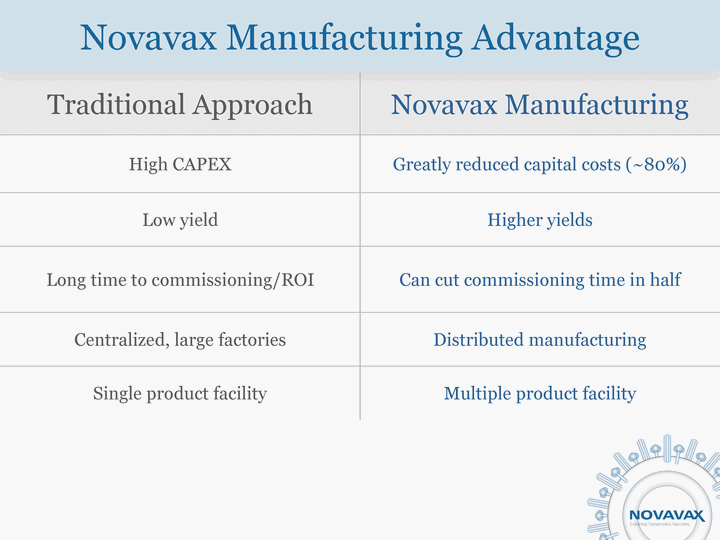
| Traditional Approach Novavax Manufacturing High CAPEX Greatly reduced capital costs (~80%) Low yield Higher yields Long time to commissioning/ROI Can cut commissioning time in half Centralized, large factories Distributed manufacturing Single product facility Multiple product facility Novavax Manufacturing Advantage |
| Flexible Commercialization Strategy 10MM dose capacity for seasonal influenza vaccine in place (Rockville, MD) Advanced manufacturing knowledge & capacity is valuable to potential partner (Corporate or Government) Economically viable regional or in-border manufacturing solution (e.g. with GE Healthcare) particularly valuable for certain targets, e.g. Pandemic flu, regional diseases Direct Product Sale Partnering Regional Manufacturing |

| Sound Financial Position NASDAQ: NVAX Shares outstanding: 69 million Current market capitalization (10/28/08): ~$150 million Cash, equivalent and short term (6/30/08): $36 million (+$18M equity round) 2008 burn: ~$8 million/quarter run rate Financing options: Partnering; non-dilutive and equity |

| 100+ Years of Vaccine Development Experience John Lambert Chairman of the Board 30 years in vaccines, Former President Chiron Vaccines, Former President APMSD (Aventis/Merck joint venture) Rahul Singhvi, ScD, MBA President and CEO 14 years in vaccines (Merck, Novavax) Penny Heaton, MD Chief Medical Officer and VP Development 7 years with Merck Vaccines; rotavirus vaccine development leader Gale Smith, PhD VP Vaccine Research 30+ years experience in biotech/vaccines; baculovirus system inventor Jim Robinson VP Technical & Quality Operations 20+ years in process development and manufacturing with Sanofi Pasteur Other Executive Committee Members Len Stigliano, VP, CFO; Ray Hage, SVP, Commercial Operations; Tom Johnston, VP, Strategy |
| Why Novavax? Proven technology used in vaccine development Strong IP for VLP franchise Strong proof of concept clinical data Broad application of VLPs to multiple virus candidates Unique and efficient manufacturing process Key clinical milestones in near term Potential blockbuster vaccines in discovery |

| BIO Investor Forum 2008 San Francisco, CA 30 October, 2008 |