Exhibit 99.1

| Corporate Presentation January, 2008 Nasdaq: NVAX |

| "Safe Harbor" Statement Statements herein relating to future financial or business performance, conditions or strategies and other financial and business matters, including expectations regarding clinical development, product sales, operating expenses, and anticipated milestones are forward-looking statements within the meaning of the Private Securities Litigation Reform Act. Novavax cautions that these forward-looking statements are subject to numerous assumptions, risks and uncertainties, which change over time. Factors that may cause actual results to differ materially from the results discussed in the forward- looking statements or historical experience include risks and uncertainties, including Novavax's ability to progress any product candidates in pre-clinical or clinical trials; the scope, rate and progress of its pre-clinical trials and other research and development activities; the scope, rate and progress of any clinical trials we commence; clinical trial results; risks relating to the early stage of Novavax's product candidates under development; even if the data from pre-clinical or clinical trials is positive, the product may not prove safe and efficacious; Novavax's pilot plant facility is subject to extensive validation and FDA inspections, which may result in delays and increased costs; the failure by Novavax to secure and maintain relationships with collaborators; uncertainties relating to clinical trials; risks relating to the commercialization, if any, of Novavax's proposed product candidates; dependence on the efforts of third parties; dependence on intellectual property; competition for clinical resources and patient enrollment from drug candidates in development by other companies with greater resources and visibility; and risks that we may lack the financial resources and access to capital to fund our operations. Further information on the factors and risks that could affect Novavax's business, financial conditions and results of operations, is contained in Novavax's filings with the U.S. Securities and Exchange Commission, which are available at http://www.sec.gov. These forward-looking statements speak only as of the date of this presentation, and Novavax assumes no duty to update forward-looking statements. |

| Discussion Points The New Novavax New Term Milestones Our Strategy to Succeed VLP/Manufacturing Technology Product Pipeline Team Summary |
| The New Novavax Clinical stage vaccine development company based in Rockville, MD NASDAQ: NVAX Shares Outstanding: 62 Million Current Cash (9/30/07): $52 Million Institutional Ownership* 46% Stock Float 90% * as of Sept 30, 2007 |

| Vaccine Market Growth Drivers Existing Vaccine bar graph Worldwide Vaccine Growth (Datamonitor, Kalorama, Novavax) Large, growing markets Expanding government recommendations for immunizations New indications Technological innovations New differentiated products for unmet needs Higher prices/margins Double growth rate projected vs. traditional therapeutics |

| Disease Unmet Medical Need Potential Market (1) RSV No current vaccine >$1bn VZV 1st Generation product >$1bn S Aureus No current vaccine $1.8bn* Flu Vaccine Elderly differentiated product >$1bn Pneumococcal 1st Generation Product $2.2bn* Mening B No current vaccine $1.8bn* Colorectal No current vaccine >$2.5bn Melanoma No current vaccine >$1.5bn Example of New Vaccines-Market Potential (1) Peak annual sales * Datamonitor for YR2016 ** Datamonitor, Oncovision v3.0, EpiVision Oncology 2003 |

| Our Strategy to Succeed Vaccine Technologies Product Pipeline Management Team |

| Basic VLPs Target Surface Proteins Lipid Envelope Novavax VLPs Single Protein Crystalline VLP e.g. Gardasil(r) (HPV vaccine) e.g. Influenza vaccine Virus Like Particle (VLP) Vaccine Technology NOVAVAX Enhanced VLP platform VLP is a proven technology for creating safe and efficacious vaccines |
| Novavax VLP: Why It Matters VLPs are "decoys", made of proteins presented in the 3-D conformation native to the targeted viruses Induce immune responses similar to natural infection Stimulate multiple arms of the immune system Potential safety advantages: No genetic machinery No adjuvant required Manufacturing efficiencies Novavax VLP vaccines have immunologic and safety advantages |
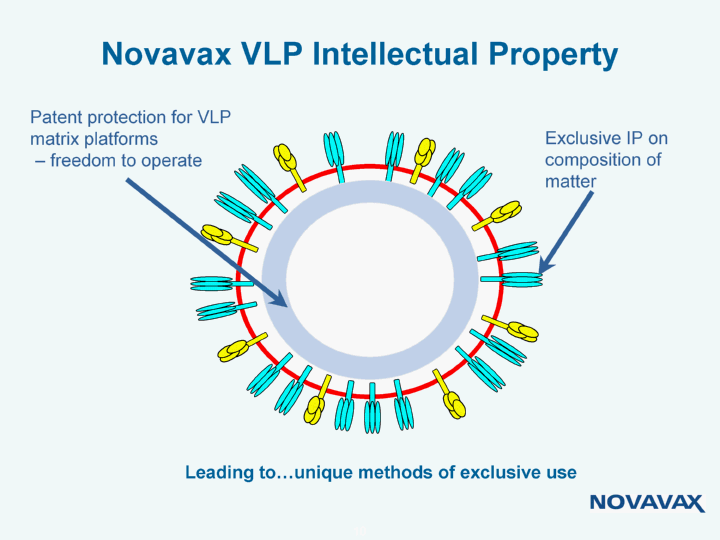
| Novavax VLP Intellectual Property Exclusive IP on composition of matter Patent protection for VLP matrix platforms - freedom to operate Leading to...unique methods of exclusive use |

| Manufacturing Excellence is Key for Success in Vaccines Traditional Vaccine VLP Vaccines 70,000 eggs ^ 100 liters |

| Disposable Manufacturing Solution Leads To......... Lower capital costs Shorter commissioning time Improved manufacturing yields & quality (cost/dose) Lower production risks (e.g. contamination) Synergies of a single production platform |

| Manufacturing Advantages Plant Build Costs/Space Facility $150,000,000 Sq ft 145,000 Facility $40,000,000 Sq ft 40,000 NVAX VLPs Egg Based Seasonal Flu Vaccine Example: 100 Million Doses Annual Capacity |
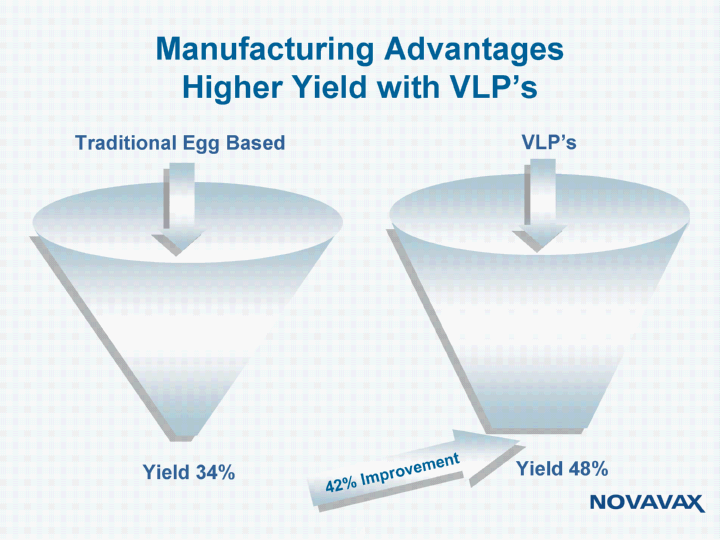
| Manufacturing Advantages Higher Yield with VLP's Traditional Egg Based Yield 34% Yield 48% VLP's 42% Improvement |
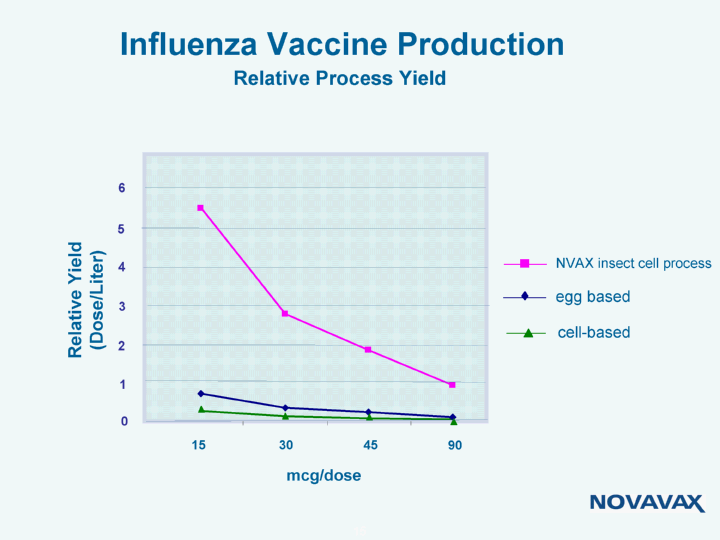
| Influenza Vaccine Production Relative Process Yield 15 30 45 90 mcg/dose Relative Yield (Dose/Liter) egg based NVAX insect cell process cell-based 3 4 5 6 0 2 1 |

| Pipeline: Leveraging the VLP Platform Discovery Preclinical Phase I/IIA Phase IIb/III Pandemic influenza Zoster (Shingles) Undisclosed Seasonal influenza HIV (with UAB) Dengue (with U. Pitt) Funded by NIH E-selectin (with NIH) Funded Programs Funded by DOD |
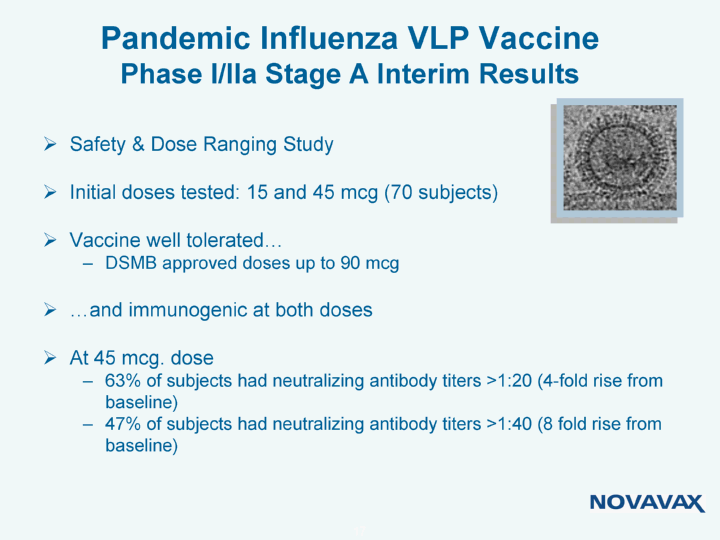
| Pandemic Influenza VLP Vaccine Phase I/IIa Stage A Interim Results Safety & Dose Ranging Study Initial doses tested: 15 and 45 mcg (70 subjects) Vaccine well tolerated... DSMB approved doses up to 90 mcg ...and immunogenic at both doses At 45 mcg. dose 63% of subjects had neutralizing antibody titers >1:20 (4-fold rise from baseline) 47% of subjects had neutralizing antibody titers >1:40 (8 fold rise from baseline) |
| Implications from Interim Results Promising recombinant H5N1 vaccine without an adjuvant that induces similar immunogenicity (at 45mcg dose) as licensed vaccine and that can be produced within 12 weeks Higher doses being tested in second stage of study Proof of Concept for Novavax VLP technology Increased probability of success for seasonal flu VLP vaccine program Safety data from H5N1 useful for seasonal flu program |
| Pandemic Influenza Opportunity "Everything we say before a pandemic will be viewed as alarmist, everything we do after it happens will be insufficient"* Insufficient worldwide vaccine capacity Globally, government budgets for pandemic preparedness exceed $13 Billion USD Market segments include: stockpile, capacity pre-purchase, domestic supply through local infrastructure Several challenges persist: Substantial gap in ability to supply strain-matched vaccine for first wave Government desire for more affordable in-country vaccine infrastructure *Tommy Thompson, US Secretary Human Health Services |

| Pandemic Influenza Customer Segments Possible strain mismatch Limited population coverage Replenishment of aged doses Subcontractor supply role to large pharma Long production lead time Cross-border shipments 12 week response time with strain match Domestic infrastructure Long production lead time Substantial Capital Expenditure investment 12 week response time with strain match Reduced Capital Expenditure Supply chain control Stockpile Capacity Pre-purchase Domestic Infrastructure Plan Example Countries Challenges Novavax Opportunity USA USA UK UK GE ES JP SWE IND TW ROK IT FR CHN UK SWE ES NL JP GE IT FR AUS AUS NL CA Evolution of Preparedness AUS |

| Domestic Infrastructure Segment Detail Substantial capacity lacking in most advanced countries All countries lack first wave protection through rapid delivery of a strain matched vaccine Existing Planned AUS USA GE ES JP SWE IND TW ROK IT FR UK NL CA CHN TW BR TH SG RSA IDN Supply Gap> 10bn doses* * Oliver Wyman/Path - Influenza Vaccine Strategies for Broad Global Access Nov 2007 Sufficient Capacity In-sufficient Capacity |

| Pandemic Vaccine Competition & Capabilities Novavax offers a complete pandemic vaccine solution Attributes Novavax Competition Remarks Efficacy Immunogenicity similar to approved vaccine; others unapproved Safety No Adjuvants, proven technology, No wild type virus Response Time 12 weeks vs 6 months Regulatory Path No Adjuvants, proven technology Scale up efficiency Lower Capital expenditure, shorter commissioning Yield (dose/L) No Eggs, COGS ok at high doses, no adjuvants Domestic infrastructure Distributed manufacturing |

| Novavax Vaccine Value Proposition = First Wave + Domestic Infrastructure Multiple potential commercial channels Traditional (RFP) and non-traditional (GE) Existing Planned AUS USA GE ES JP SWE IND TW ROK IT FR UK NL CA CHN TW BR TH SG RSA IDN Supply Gap > 10bn doses* * Oliver Wyman/Path - Influenza Vaccine Strategies for Broad Global Access Nov 2007 Sufficient Capacity In-sufficient Capacity + First Wave |
| Novavax and GE Collaboration: A New Business Model Step 1: Co-Market a pandemic influenza vaccine solution, based on Novavax's VLP vaccine and manufacturing platform using GE products, to select international countries Step 2: Execute an agreement with a target country to enable domestic vaccine production infrastructure Step 3: Implement commercialization within country Step 4 (in parallel to Step 2 and 3) Repeat process with other select countries |

| Seasonal Influenza VLP Vaccine Large, growing market with unmet medical needs US Government plans to expand current vaccine recommendations for vaccination 2010 - 2012 Need for a more immunogenic vaccine in older adults Two Pronged Development Strategy Rapidly develop a non-inferior recombinant product without adjuvant using defined regulatory pathway In parallel, test efficacy in older adults for superiority vs. standard care Probability of success enhanced by interim H51 data Near-term Development Timelines Complete remaining pre-clinical studies end of Q1 2008 Commence human clinical trials Q2 2008 |

| Varicella Zoster Virus (VZV) Vaccine VZV is responsible for over 1 million cases of herpes zoster (shingles) in the US each year Primary complication is post-herpetic neuralgia (PHN), which occurs in ~65% of affected patients Large market with only one vaccine supplier Current vaccine provides ~50% efficacy against shingles and ~39% efficacy against PHN Potential advantages of Novavax's VZV vaccine (i.e. not a live virus as current marketed product). |

| The Talent And Experience To Succeed John Lambert: Chairman of the Board 30 years in vaccines, Former President Chiron Vaccines, Former President APMSD (Aventis/Merck joint venture) Rahul Singhvi ScD MBA: President and CEO 10 years with Merck vaccines Penny Heaton MD: Chief Medical Officer and VP Development 7 years with Merck vaccines; rotavirus vaccine development leader Gale Smith PhD: VP Vaccine Research CSO Protein Sciences; baculovirus system inventor Jim Robinson: VP Technical & Quality Operations 20+ years in process development and manufacturing with Sanofi Pasteur Raymond Hage: SVP Commercial Operations 15 years in sales, marketing and business development including 10 yrs at Lilly Len Stigliano: CFO 30+ years in life sciences; financial and general management roles |

| Key 2008 Milestones Top line data for Pandemic flu Phase I/IIa trial with final dose Q2 2008 Commence seasonal flu Phase I/IIa trial Q2 2008 Obtain Top line data from seasonal flu Phase I/IIa trial Q3 2008 Obtain initial pre-clinical results for VZV Vaccine Q3 2008 Initiate Divestiture of Legacy Assets (cash) TBD Seek non-dilutive funding for Pandemic Flu program TBD |

| Key Takeaways A proven recombinant vaccine approach with a broad platform Focus on products with clear development and regulatory approval pathway Two vaccines (pandemic and seasonal) through Phase IIa by Q3'08 Two novel, high value vaccine programs in early development Cost effective, paradigm changing manufacturing technology Novel commercialization strategy with GE Healthcare Experienced vaccine development management team Significant value creating opportunity in 2008 |

| Nasdaq: NVAX |