Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
Filed by the Registrant x Filed by a Party Other Than the Registrant ¨
Check the Appropriate Box:
| ¨ | Preliminary Proxy Statement | |||
| ¨ | Confidential, for Use of the Commission Only (as Permitted by Rule 14a-6(e)(2)) | |||
| x | Definitive Proxy Statement | |||
| ¨ | Definitive Additional Materials | |||
| ¨ | Soliciting Material Pursuant to §240.14a-12 | |||
| Pfizer Inc. | ||||
| (Name of Registrant as Specified In Its Charter) | ||||
| (Name of Person(s) Filing Proxy Statement, if other than the Registrant) | ||||
| Payment of filing fee (Check the appropriate box): | ||||
| x | No fee required | |||
| ¨ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11 | |||
| (1) | Title of each class of securities to which transaction applies:
| |||
|
| ||||
| (2) | Aggregate number of securities to which transaction applies:
| |||
|
| ||||
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined):
| |||
|
| ||||
| (4) | Proposed maximum aggregate value of transaction:
| |||
|
| ||||
| (5) | Total fee paid: | |||
|
| ||||
| ¨ | Fee paid previously with preliminary materials. | |||
| ¨ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. | |||
| (1) | Amount Previously Paid:
| |||
|
| ||||
| (2) | Form, Schedule or Registration Statement No.:
| |||
|
| ||||
| (3) | Filing Party:
| |||
|
| ||||
| (4) | Date Filed:
| |||
|
| ||||
Table of Contents

| 1 | The 2011 Financial Report is not included in this filing. The portions of the 2011 Financial Report that are incorporated by reference in our Annual Report on Form 10-K for the fiscal year ended December 31, 2011 (the “2011 Form 10-K”) were filed, and the other portions of the 2011 Financial Report were furnished, solely for the information of the SEC on Exhibit 13 to the 2011 Form 10-K. The 2011 Financial Report is contained in Appendix A to the Proxy Statement being mailed to our shareholders beginning on or about March 15, 2012. The Letter to Shareholders and Corporate and Shareholder Information contained in the materials being mailed to our shareholders beginning on or about March 15, 2012 are not included in this filing. |
Table of Contents
HOW TO VOTE
Most shareholders have a choice of voting on the Internet, by telephone, or by mail using a traditional proxy card. Please refer to the proxy card or other voting instructions included with these proxy materials for information on the voting method(s) available to you. If you vote by telephone or on the Internet, you do not need to return your proxy card.
ANNUAL MEETING ADMISSION
Either an admission ticket or proof of ownership of Pfizer stock, as well as a form of personal photo identification, must be presented in order to be admitted to the Annual Meeting. If you are a shareholder of record, your admission ticket is attached to your proxy card or the “Notice of Internet Availability of Proxy Materials” referred to below. If your shares are held in the name of a broker, bank or other holder of record, you must bring a brokerage statement or other proof of ownership with you to the Meeting, or you may request an admission ticket in advance. For further details, please see “Do I need a ticket to attend the Annual Meeting?” under “Proxy Statement—Questions and Answers About the Annual Meeting and Voting.”
NOTICE AND ACCESS; ELECTRONIC DELIVERY OF PROXY MATERIALS
This year, we are pleased to be distributing our proxy materials to certain shareholders via the Internet under the “notice and access” approach permitted by rules of the Securities and Exchange Commission. This approach conserves natural resources and reduces our costs of printing and distributing the proxy materials, while providing a convenient way to access the materials and vote. On March 15, 2012, we mailed a “Notice of Internet Availability of Proxy Materials” to participating shareholders, containing instructions on how to access the proxy materials on the Internet.
Shareholders who are not participating in notice and access can nonetheless help us reduce costs and conserve resources by opting to receive future proxy materials electronically. Shareholders of record may enroll in the electronic proxy delivery service at any time by going directly to www.computershare-na.com/green. Beneficial owners should contact their broker, bank or other holder of record regarding the availability of this service. We encourage all of our shareholders to consider this option and help us conserve resources and reduce expenses.
HOUSEHOLDING
If you and other Pfizer shareholders living in your household do not receive your proxy materials electronically, you may opt to receive only one copy of future proxy statements and financial reports. Please see “What is ‘householding’ and how does it affect me?” under “Proxy Statement—Questions and Answers about the Annual Meeting and Voting” for more information on this important shareholder program.
PFIZER’S ANNUAL REVIEW AVAILABLE ONLINE
Since Pfizer is working hard to be a greener company, we no longer print paper copies of the Pfizer Annual Review to Shareholders. If you would like to view the Annual Review online, visit www.pfizer.com/annual.
Table of Contents
Proxy Summary
Here are highlights of important information you will find in this Proxy Statement. As it is only a Summary, please review the complete Proxy Statement before you vote.
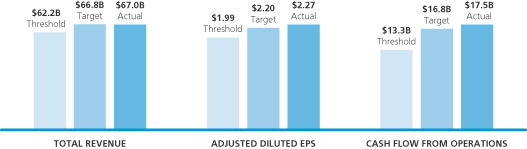
| PFIZER 2011 COMPANY HIGHLIGHTS |
For additional information about Pfizer, please view our 2011 Annual Review at www.pfizer.com/annual
| 2012 PROXY STATEMENT |

|
i |
Table of Contents
PROXY SUMMARY
| ii |

|
2012 PROXY STATEMENT |
Table of Contents
PROXY SUMMARY
| SHAREHOLDER VOTING MATTERS |
SUMMARY OF SHAREHOLDER VOTING MATTERS
| VOTING MATTER | BOARD VOTE RECOMMENDATION | SEE PAGE NUMBER for more information | ||
| Item 1 Election of Directors |
FOR each nominee |
23 | ||
| Item 2 Ratification of Independent Registered Public Accounting Firm |
FOR |
31 | ||
| Item 3 Advisory Approval of Executive Compensation |
FOR |
34 | ||
| Shareholder Proposals:
Item 4 Publication of Political Contributions
Item 5 Action by Written Consent
Item 6 Special Shareholder Meetings
Item 7 Advisory Vote on Director Pay |
AGAINST
AGAINST
AGAINST
AGAINST |
36
37
38
39 | ||
| OUR DIRECTOR NOMINEES |
You are being asked to vote on these 14 Directors. All Directors are elected annually by a majority of votes cast. Detailed information about each Director’s background, skill sets and areas of expertise can be found beginning on page 24.
| NAME | AGE | DIRECTOR SINCE |
POSITION | INDE- PENDENT |
COMMITTEE MEMBERSHIPS | OTHER CURRENT PUBLIC BOARDS | ||||||||||||||
| AC | CC | CGC | RCC | STC | ||||||||||||||||
| Dennis A. Ausiello, M.D. |
66 | 2006 | Professor, Harvard Medical School and Chief of Medicine, Massachusetts General Hospital |
Yes | M | M | M | M | – | |||||||||||
| M. Anthony Burns | 69 | 1988 | Chairman Emeritus, Ryder System, Inc. |
Yes | M | M | 1 | |||||||||||||
| W. Don Cornwell | 64 | 1997 | Former Chairman and CEO, Granite Broadcasting |
Yes | C | M | M | 2 | ||||||||||||
| Frances D. Fergusson, Ph.D. | 67 | 2009 | President Emeritus, Vassar College |
Yes | M | C | M | 1 | ||||||||||||
| William H. Gray, III | 70 | 2000 | Chairman of Gray Global Strategies |
Yes | C | M | 3 | |||||||||||||
| Helen H. Hobbs, M.D. | 59 | 2011 | Investigator, Howard Hughes Medical Institute and Professor, University of Texas Southwestern Medical Center |
Yes | M | M | – | |||||||||||||
| Constance J. Horner | 70 | 1993 | Former Assistant to the President of the United States and Director of Presidential Personnel |
Yes | M | M | 2 | |||||||||||||
| James M. Kilts | 64 | 2007 | Founding Partner, Centerview Capital | Yes | C | M | 3 | |||||||||||||
| George A. Lorch | 70 | 2000 | Chairman Emeritus, Armstrong Holdings, Inc. |
LEAD INDE- PENDENT |
2 | |||||||||||||||
| John P. Mascotte | 72 | 2009 | Retired President & CEO, Blue Cross and Blue Shield of Kansas City |
Yes | M | M | M | – | ||||||||||||
| Suzanne Nora Johnson | 54 | 2007 | Retired Vice Chairman, Goldman Sachs Group |
Yes | M | M | M | 3 | ||||||||||||
| Ian C. Read | 58 | 2010 | Chairman & CEO, Pfizer | No | 1 | |||||||||||||||
| Steven W. Sanger | 66 | 2009 | Former Chairman & CEO, General Mills |
Yes | M | M | 2 | |||||||||||||
| Marc Tessier-Lavigne, Ph.D. | 52 | 2011 | President, Rockefeller University; former EVP and Chief Scientific Officer, Genentech |
Yes | M | M | 1 | |||||||||||||
| AC | Audit Committee | RCC | Regulatory and Compliance Committee | C |
Chair | |||||
| CC | Compensation Committee | STC |
Science and Technology Committee | M |
Member | |||||
| CGC | Corporate Governance Committee |
|||||||||
| 2012 PROXY STATEMENT |

|
iii |
Table of Contents
PFIZER INC.
235 East 42nd Street
New York, New York 10017-5755
NOTICE OF 2012 ANNUAL MEETING OF SHAREHOLDERS
| TIME AND DATE |
8:30 a.m., Eastern Daylight Time, on Thursday, April 26, 2012. |
| PLACE |
Westin Governor Morris |
| 2 Whippany Road |
| Morristown, New Jersey 07960 |
| WEBCAST |
A webcast of our Annual Meeting will be available on our website, www.pfizer.com, starting at 8:30 a.m., Eastern Daylight Time, on Thursday, April 26, 2012. An archived copy of the webcast will be available on our website through the first week of May 2012. Information included on our website, other than our Proxy Statement and form of proxy, is not a part of our proxy solicitation materials. |
| ITEMS OF BUSINESS |
• | To elect 14 members of the Board of Directors named in the Proxy Statement, each for a term of one year. |
| • | To ratify the appointment of KPMG LLP as our independent registered public accounting firm for the 2012 fiscal year. |
| • | To conduct an advisory vote to approve our executive compensation. |
| • | To consider certain shareholder proposals, if presented at the Meeting; see the Table of Contents for further information. |
| • | To transact any other business that properly comes before the Meeting and any adjournment or postponement of the Meeting. |
| RECORD DATE |
You can vote if you were a shareholder of record at the close of business on February 28, 2012. |
| MATERIALS TO REVIEW |
This booklet contains our Notice of 2012 Annual Meeting and Proxy Statement. Our 2011 Financial Report is in Appendix A to this Notice of Annual Meeting and Proxy Statement and is followed by certain Corporate and Shareholder Information. Appendix A and the Corporate and Shareholder Information, as well as the accompanying Letter to Shareholders, are not a part of our proxy solicitation materials. You may also access these documents through our website at www.pfizer.com/annualmeeting. |
| PROXY VOTING |
It is important that your shares be represented and voted at the Meeting. You can vote your shares by completing and returning your proxy card or by voting on the Internet or by telephone. See details under “How do I vote?” under “Proxy Statement—Questions and Answers About the Annual Meeting and Voting.” |
| IMPORTANT NOTICE REGARDING THE AVAILABILITY OF PROXY MATERIALS FOR THE ANNUAL MEETING OF SHAREHOLDERS TO BE HELD ON APRIL 26, 2012: This Notice of Annual Meeting and Proxy Statement and the 2011 Financial Report and Corporate and Shareholder Information are available on our website at www.pfizer.com/annualmeeting. |
Matthew Lepore
Corporate Secretary
March 15, 2012
Table of Contents
| 1 | ||||
| 5 | ||||
| 5 | ||||
| 5 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| 8 | ||||
| 9 | ||||
| 10 | ||||
| 10 | ||||
| 11 | ||||
| 11 | ||||
| 12 | ||||
| 13 | ||||
| 13 | ||||
| 15 | ||||
| 16 | ||||
| 16 | ||||
| 17 | ||||
| 18 | ||||
| 21 | ||||
| SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE, RELATED PERSON TRANSACTIONS, AND INDEMNIFICATION | 22 | |||
| 23 | ||||
| 23 | ||||
| 24 | ||||
| Item 2—Ratification of Independent Registered Public Accounting Firm |
31 | |||
| 31 | ||||
| 31 | ||||
| 32 | ||||
| 34 | ||||
| 36 | ||||
| Item 4—Shareholder Proposal regarding Publication of Political Contributions |
36 | |||
| Item 5—Shareholder Proposal regarding Action by Written Consent |
37 | |||
| Item 6—Shareholder Proposal regarding Special Shareholder Meetings |
38 | |||
| Item 7—Shareholder Proposal regarding Advisory Vote on Director Pay |
39 | |||
| 41 | ||||
| 42 | ||||
| 46 | ||||
| 47 | ||||
| 70 | ||||
| 79 | ||||
| 80 | ||||
| 81 | ||||
| REQUIREMENTS, INCLUDING DEADLINES, FOR SUBMISSION OF PROXY PROPOSALS AND NOMINATION OF DIRECTORS | 82 | |||
| 83 | ||||
| i | ||||
| Inside Back Cover | ||||

|
2012 PROXY STATEMENT |
Table of Contents
| Pfizer Inc. 235 East 42nd Street New York, New York 10017-5755 |
Proxy Statement
| QUESTIONS AND ANSWERS ABOUT THE ANNUAL MEETING AND VOTING |
| 2012 PROXY STATEMENT |

|
1 |
Table of Contents
PROXY STATEMENT
| 2 |

|
2012 PROXY STATEMENT |
Table of Contents
PROXY STATEMENT
| 2012 PROXY STATEMENT |

|
3 |
Table of Contents
PROXY STATEMENT
| 4 |

|
2012 PROXY STATEMENT |
Table of Contents
| 2012 PROXY STATEMENT |

|
5 |
Table of Contents
GOVERNANCE OF THE COMPANY
| 6 |

|
2012 PROXY STATEMENT |
Table of Contents
GOVERNANCE OF THE COMPANY
| 2012 PROXY STATEMENT |

|
7 |
Table of Contents
GOVERNANCE OF THE COMPANY
| 8 |

|
2012 PROXY STATEMENT |
Table of Contents
GOVERNANCE OF THE COMPANY
In addition, the independent Directors considered shareholder feedback on the subject of Board leadership, including discussions with institutional investors who expressed interest in learning more about the Board’s rationale for recombining the roles of Chairman and CEO. In general, these investors acknowledge that the independent members of the Board are in the best position to determine the optimal Board structure, although some investors expressed concern about the strength of board independence under a non-independent chair structure. Further, our investors indicated that if the positions of Chairman and CEO should be combined, it was imperative that the Board have independent leadership by appointing a strong Lead Independent Director. The Company’s Corporate Governance Principles require the appointment of a Lead Independent Director if the positions of Chairman and CEO are held by the same individual, and the independent Directors believe that Mr. Lorch provides strong leadership in that position.
While Pfizer’s independent Directors are aware of investor concerns regarding our Board leadership structure, they believe that our Board, comprised entirely of independent Directors other than Mr. Read, remains highly independent, empowered and engaged. Further, the independent Directors remain committed to evaluating our Board leadership structure at least annually. Under the Company’s By-laws and Corporate Governance Principles, the Board can and will change its leadership structure if it determines that doing so is appropriate and in the best interest of Pfizer and its shareholders. The Board believes that these factors provide the appropriate balance between the authority of those who oversee the Company and those who manage it on a day-to-day basis.
Lead Independent Director
The position of Lead Independent Director at Pfizer comes with a clear mandate and significant authority and responsibilities under a Board-approved Charter, including the following:
| • | presiding at executive sessions of the independent Directors; |
| • | calling meetings of the independent Directors; |
| • | serving as liaison between the independent Directors and the Chairman; |
| • | approving information sent to the Board, including the quality, quantity and timeliness of such information; |
| • | approving meeting agendas; |
| • | facilitating the Board’s approval of the number and frequency of Board meetings and approving meeting schedules, to assure that there is sufficient time for discussion of all agenda items; |
| • | authorizing the retention of outside advisors and consultants who report directly to the Board; and |
| • | if requested by shareholders, ensuring that he/she is available, when appropriate, for consultation and direct communication. |
The Charter of the Lead Independent Director can be found on our website at http://www.pfizer.com/about/corporate_governance/lead_independent_charters.jsp.
Until April 2011, the Board included one non-employee Director who was not considered independent. During his tenure, executive sessions of the non-employee Directors took place at least four times each year, and the independent Directors met in executive session at least once each year. Since then, the Board has been composed entirely of independent Directors, except for Mr. Read, and executive sessions of the independent Directors have generally taken place at every regular Board meeting. At these executive sessions, the independent Directors review, among other things, the criteria upon which the performance of the Chief Executive Officer and other senior managers is evaluated, the performance of the Chief Executive Officer against those criteria, and the compensation of the Chief Executive Officer and other senior managers.
| 2012 PROXY STATEMENT |

|
9 |
Table of Contents
GOVERNANCE OF THE COMPANY
The Board’s Role in Risk Oversight
Management is responsible for assessing and managing risk, subject to oversight by the Board. The Board executes its oversight responsibility for risk assessment and risk management directly and through its Committees, as follows:
| • | The Audit Committee has primary responsibility for overseeing the Company’s Enterprise Risk Management, or “ERM,” program. The Company’s Chief Internal Auditor, who reports to the Committee, facilitates the ERM program, in coordination with the Company’s Legal Division and Compliance Group, to complement the Company’s strategic planning process. The Committee’s meeting agendas throughout the year include discussions of individual risk areas, as well as an annual summary of the ERM process. For additional information, see “Board and Committee Information—The Audit Committee” and “Item 2—Ratification of Independent Registered Public Accounting Firm—Audit Committee Report” later in this Proxy Statement. |
| • | The Regulatory and Compliance Committee, formed in February 2011, has primary responsibility for overseeing and reviewing risks associated with the Company’s healthcare law compliance programs and the status of compliance with related laws, regulations and internal procedures. The Committee, in consultation with the Compensation Committee, is responsible for discussing with management the risks associated with our compensation policies and practices for sales and marketing personnel and the alignment of compensation practices with the Company’s compliance standards. For additional information, see “Board and Committee Information—The Regulatory and Compliance Committee” later in this Proxy Statement. |
| • | The Board’s other Committees—Compensation, Corporate Governance, and Science and Technology—oversee risks associated with their respective areas of responsibility. For example, the Compensation Committee considers the risks associated with our compensation policies and practices, with respect to both executive compensation and compensation generally. |
| The Board of Directors is kept informed of its Committees’ risk oversight and other activities through reports of the Committee Chairmen to the full Board. These reports are presented at every regular Board meeting and include discussions of Committee agenda topics, including matters involving risk oversight. |
| • | The Board considers specific risk topics, including risks associated with our strategic plan, our capital structure and our development activities. In addition, the Board receives regular reports from the members of our Executive Leadership Team, or “ELT”—the heads of our principal business and corporate functions—that include discussions of the risks and exposures involved in their respective areas of responsibility. These reports are provided in connection with and discussed at Board meetings. At other times, the Board is routinely informed of developments that could affect our risk profile or other aspects of our business. |
Pfizer Policies on Business Ethics and Conduct
All of our employees, including our Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer, are required to abide by Pfizer’s policies on business conduct to ensure that our business is conducted in a consistently legal and ethical manner. Pfizer’s policies form the foundation of a comprehensive process that includes compliance with corporate policies and procedures, an open relationship among colleagues that contributes to good business conduct, and a high level of integrity. Our policies and procedures cover all major areas of professional conduct, including employment practices, conflicts of interest, intellectual property and the protection of confidential information, and require strict adherence to laws and regulations applicable to the conduct of our business.
Employees are required to report any conduct that they believe in good faith to be an actual or apparent violation of Pfizer’s policies on business conduct. As required by the Sarbanes-Oxley Act of 2002, our Audit Committee has procedures to receive, retain and treat complaints received regarding accounting, internal accounting controls or auditing matters and to allow for the confidential and anonymous submission by employees of concerns regarding questionable accounting or auditing matters.
To review Pfizer’s Summary of Policies on Business Conduct, please visit our website at
http://www.pfizer.com/about/corporate_compliance/code_of_conduct.jsp.
| 10 |

|
2012 PROXY STATEMENT |
Table of Contents
GOVERNANCE OF THE COMPANY
| 2012 PROXY STATEMENT |

|
11 |
Table of Contents
GOVERNANCE OF THE COMPANY
The Company’s relationships with its shareholders and other stakeholders are a critical part of our corporate governance profile, and we recognize the value of taking their views into account. Among other things, engagement with our shareholders and other stakeholders helps us to understand the larger context and impact of our operations, learn about expectations for our performance, assess emerging issues that may affect our business or other aspects of our operations, and shape corporate and governance policies. Over the years, this approach has helped us to identify mutual perspectives and goals and to adopt a collaborative approach to these relationships, and has resulted in our receiving essential input from shareholders and other stakeholders.
For example, in the wake of the United States Supreme Court’s 2010 decision in Citizens United vs. The Federal Election Commission, we engaged in extensive discussions with shareholders and stakeholders seeking clarification about Pfizer’s policies on corporate political expenditures. These discussions led to our decision in 2011 to adopt a strict policy against Pfizer making “independent expenditures” in connection with any federal or state election.
Additional information regarding Pfizer’s political contributions can be found at
http://www.pfizer.com/responsibility/grants_contributions/lobbying_and_political_contributions.jsp.
In addition, consistent with Pfizer’s commitment to seek and respond to shareholder input on corporate governance topics, we have considered and discussed with investors a wide variety of matters, including our executive compensation program and disclosures, and have made a number of changes in both areas. See “Compensation Discussion and Analysis” elsewhere in this Proxy Statement.
| 12 |

|
2012 PROXY STATEMENT |
Table of Contents
GOVERNANCE OF THE COMPANY
BOARD AND COMMITTEE INFORMATION
During 2011, the Board of Directors met eight times and had five key Committees: the Audit Committee, the Compensation Committee, the Corporate Governance Committee, the Regulatory and Compliance Committee, and the Science and Technology Committee. Each of our Directors attended at least 94% of the meetings of the Board and the Board Committees on which he or she served that were held during the time he or she was a Director in 2011.
All Board members are expected to attend the Annual Meeting unless an emergency prevents them from doing so. All the Directors then in office attended our 2011 Meeting.
The table below provides membership and meeting information for each of the Board Committees for 2011.
| NAME | AUDIT | COMPENSATION | CORPORATE GOVERNANCE |
REGULATORY AND COMPLIANCE |
SCIENCE AND TECHNOLOGY | |||||||||||||
| Dr. Ausiello |
X | X | X | X | ||||||||||||||
| Dr. Brown(a) |
X | X* | ||||||||||||||||
| Mr. Burns |
X | X | ||||||||||||||||
| Mr. Burt(b) |
X | X | ||||||||||||||||
| Mr. Cornwell |
X* | X | X | |||||||||||||||
| Dr. Fergusson |
X | X* | X | |||||||||||||||
| Mr. Gray |
X* | X | ||||||||||||||||
| Dr. Hobbs(c) |
||||||||||||||||||
| Ms. Horner |
X | X | ||||||||||||||||
| Mr. Kilts |
X* | X | ||||||||||||||||
| Mr. Lorch(d) |
||||||||||||||||||
| Mr. Mascotte |
X | X | X | |||||||||||||||
| Ms. Nora Johnson |
X | X | X | |||||||||||||||
| Mr. Read |
||||||||||||||||||
| Mr. Sanger |
X | X | ||||||||||||||||
| Mr. Steere(e) |
X | |||||||||||||||||
| Dr. Tessier-Lavigne(c) |
||||||||||||||||||
| 2011 Meetings | 15 | 7 | 6 | 4 | 2 |
| * | Committee Chair |
| (a) | Retiring from the Board effective as of the 2012 Annual Meeting. |
| (b) | Member of the Compensation Committee and of the Science and Technology Committee until his retirement effective as of the 2011 Annual Meeting. |
| (c) | Elected on December 12, 2011. In February 2012, Dr. Hobbs joined the Corporate Governance Committee and the Science and Technology Committee, and Dr. Tessier-Lavigne joined the Regulatory and Compliance Committee and the Science and Technology Committee. |
| (d) | Served as Chairman of the Board from December 13, 2010 until December 12, 2011 and as Lead Independent Director thereafter. In both capacities, Mr. Lorch has frequently attended meetings of Committees. However, he is not a member of any Committee, in order to focus on his leadership role. |
| (e) | Member of the Science and Technology Committee until his retirement effective as of the 2011 Annual Meeting. |
The Corporate Governance Committee
The Corporate Governance Committee is comprised entirely of independent Directors and is governed by a Board-approved Charter stating its responsibilities. Under the terms of its Charter, the Committee is responsible for matters of corporate governance and matters relating to the practices, policies and procedures of the Board. This includes developing criteria for Board membership and recommending and recruiting Director candidates. The Committee also assesses Director and candidate independence, considers possible conflicts of interest of Board members and senior executives, reviews related person transactions, and monitors the functions of the various Committees of the Board.
The Committee advises on the structure of Board meetings and recommends matters for consideration by the Board. The Committee also advises on and recommends Director compensation, which is approved by the full Board. The Committee is directly responsible for overseeing the evaluation of the Board and its Committees, reviewing our Director Qualification Standards, and establishing Director retirement policies. The Committee also assists management by reviewing the functions and outside activities of senior executives and reviewing succession plans for elected corporate officers. Finally, the Committee reviews certain public policy issues, including the Company’s political spending policies and practices, as well as its regular detailed disclosures of political spending.
The Board of Directors has determined that each of the members of the Corporate Governance Committee is independent, as defined by the rules of the SEC and the NYSE, as well as under our Director Qualification Standards.
A copy of the Corporate Governance Committee Charter is available on our website at
http://pfizer.com/about/corporate_governance/corporate_governance_committee.jsp.
| 2012 PROXY STATEMENT |

|
13 |
Table of Contents
GOVERNANCE OF THE COMPANY
The Corporate Governance Committee
|
|
| |
| William H. Gray, III, Chair | Helen H. Hobbs | |
|
|
| |
| Dennis A. Ausiello | Constance J. Horner | |
|
|
| |
| Michael S. Brown | John P. Mascotte | |
|
|
| |
| M. Anthony Burns | Stephen W. Sanger | |
| 14 |

|
2012 PROXY STATEMENT |
Table of Contents
GOVERNANCE OF THE COMPANY
| 2012 PROXY STATEMENT |

|
15 |
Table of Contents
GOVERNANCE OF THE COMPANY
In its activities, the Committee considered potential risks and steps the Company has taken to mitigate risk in areas within the Committee’s oversight. With respect to the CIA, the Committee monitored the status of the Company’s compliance with CIA requirements.
The Regulatory and Compliance Committee
The Audit Committee is comprised entirely of independent Directors and is governed by a Board-approved Charter stating its responsibilities. Under its Charter, the Audit Committee is responsible for reviewing with the independent registered public accounting firm, Internal Audit and management the adequacy and effectiveness of internal controls over financial reporting. The Committee also reviews and consults with management, Internal Audit and the independent registered public accounting firm on matters related to the annual audit, the published financial statements, earnings releases, and the accounting principles applied. In addition, the Committee reviews reports from management relating to the status of compliance with laws, regulations and internal procedures.
The Committee is directly responsible for the appointment, compensation, retention and oversight of the Company’s independent registered public accounting firm. To execute this responsibility, the Committee engages in a comprehensive annual evaluation of the independent auditor’s qualifications, performance and independence and considers the advisability and potential impact of selecting a different independent public accounting firm.
In addition, the Committee is responsible for reviewing and discussing with management the Company’s policies with respect to risk assessment and risk management. Further information about the role of the Audit Committee in risk assessment and risk management is included in the section entitled “Governance Information—The Board’s Role in Risk Oversight.”
The Audit Committee has established policies and procedures for the pre-approval of all services provided by the independent auditors. The Audit Committee has also established procedures for the receipt, retention and treatment, on a confidential basis, of complaints received by the Company regarding its accounting, internal controls and auditing matters. Further details of the role of the Audit Committee may be found in “Item 2—Ratification of Independent Registered Public Accounting Firm—Audit Committee Report” later in this Proxy Statement.
The Board of Directors has determined that each of the members of the Audit Committee is financially literate and independent, as defined by the rules of the SEC and the NYSE, as well as independent under our Director Qualification Standards. The Board of Directors also has determined that each of Ms. Nora Johnson and Messrs. Burns, Cornwell and Sanger is an “audit committee financial expert” for purposes of the SEC’s rules.
A copy of the Audit Committee Charter is available on our website at http://pfizer.com/about/corporate_governance/ audit_committee.jsp. The Audit Committee Report appears under “Ratification of Independent Registered Public Accounting Firm.”
The Compensation Committee is comprised entirely of independent Directors and is governed by a Board-approved Charter stating its responsibilities. The Committee determines and oversees the execution of the Company’s executive compensation philosophy and oversees the administration of the Company’s executive compensation programs. Its responsibilities also include overseeing Pfizer’s compensation and benefit plans and policies, administering its stock plans (including reviewing and approving equity grants) and reviewing and approving annually all compensation decisions for the Company’s executive officers, including the Named Executive Officers identified in the 2011 Summary Compensation Table. See “Compensation Discussion and Analysis” later in this Proxy Statement for information concerning the Committee’s role, processes and activities in overseeing executive compensation.
| 16 |

|
2012 PROXY STATEMENT |
Table of Contents
GOVERNANCE OF THE COMPANY
The Board of Directors has determined that each of the members of the Compensation Committee is independent, as defined by the rules of the SEC and the NYSE, as well as under our Director Qualification Standards. In addition, each Committee member is a “non-employee director” as defined in Rule 16b-3 under the Securities Exchange Act of 1934, and is an “outside director” as defined in Section 162(m) of the Internal Revenue Code.
A copy of the Compensation Committee Charter is available on our website at
http://pfizer.com/about/corporate_governance/compensation_committee.jsp. The Compensation Committee Report appears under “Executive Compensation.”
Compensation Committee Interlocks and Insider Participation. During 2011 and as of the date of this Proxy Statement, none of the members of the Compensation Committee was or is an officer or employee of the Company, and no executive officer of the Company served or serves on the compensation committee or board of any company that employed or employs any member of the Company’s Compensation Committee or Board of Directors.
The Science and Technology Committee
Under the terms of its Board-approved Charter, the Science and Technology Committee is responsible for periodically examining management’s direction of and investment in the Company’s pharmaceutical research and development and technology initiatives. This includes evaluating the quality and direction of the Company’s research and development programs, identifying emerging issues and evaluating the level of review by external experts. The Committee also reviews the Company’s approaches to acquiring and maintaining technology, evaluates the technology that the Company is researching and developing and reviews the Company’s patent strategy.
A copy of the Science and Technology Committee Charter is available on our website at
http://pfizer.com/about/corporate_governance/science_ technology_committee.jsp.
| 2012 PROXY STATEMENT |

|
17 |
Table of Contents
GOVERNANCE OF THE COMPANY
COMPENSATION OF NON-EMPLOYEE DIRECTORS
Except as described below, our non-employee Directors receive cash compensation, as well as equity compensation in the form of Pfizer stock units. Each of these components is described below. The 2011 compensation of our non-employee Directors is shown in the Director Compensation Table below. Mr. Read does not receive any compensation for his service as a Director or as Chairman.
Non-Employee Director Compensation
Through April 2011, compensation for our non-employee Directors (other than Dr. Ausiello and Mr. Lorch, as discussed below) consisted of the following:
| • | an annual retainer of $75,000; and |
| • | an award of 5,500 Pfizer stock units under the Pfizer Inc. Nonfunded Deferred Compensation and Unit Award Plan for Non-Employee Directors (the “Unit Award Plan”) to each Director upon joining the Board and an award of 5,500 stock units to each Director upon election at each Annual Meeting of Shareholders, provided the Director continues to serve as a Director following the Meeting. Stock units are not payable until the Director ceases to be a member of the Board, at or after which time they are paid in cash or in shares of Pfizer stock, at the Director’s election. |
In accordance with the Unit Award Plan, on the day of the 2011 Annual Meeting of Shareholders, our non-employee Directors who continued as Directors following that Meeting (other than Dr. Ausiello and Mr. Lorch, as discussed below) were awarded 5,500 stock units with a value at the time of grant of $114,565 (calculated based on the closing stock price of Pfizer common stock of $20.83 per share on the grant date).
In addition, our compensation program for non-employee Directors provided for the following additional annual cash retainers through April 2011:
| • | Audit Committee: Chair—$25,000; Member—$20,000 |
| • | Compensation Committee: Chair—$25,000; Member—$20,000 |
| • | Corporate Governance Committee: Chair—$25,000; Member—$20,000 |
| • | Regulatory and Compliance Committee: Chair—$25,000; Member—$20,000 |
| • | Science and Technology Committee: Chair—$30,000; Senior Member—$20,000; Member—$10,000 |
| • | Lead Independent Director (if applicable): $30,000 |
Effective May 1, 2011, the Board, on the recommendation of the Corporate Governance Committee, and in consultation with the Committee’s independent compensation consultant based on a study of peers and market trends, made the following changes to the compensation program for non-employee Directors (other than Dr. Ausiello and Mr. Lorch, as discussed below):
| • | The annual cash retainer for each non-employee Director was fixed at $137,500. |
| • | The annual cash retainer for the Chair of each key Committee of the Board was fixed at $30,000. |
| • | Directors no longer receive annual cash retainers for serving as members of Committees. |
| • | The equity award to each non-employee Director upon his or her first election as such and annually thereafter was fixed at the number of units having a value of $137,500 on the date of grant (i.e., respectively, the date of his or her first election and the date of the Company’s Annual Meeting of Shareholders), based upon the closing price of Pfizer common stock on that date. |
| • | The annual cash retainer to be paid to the Lead Independent Director (if applicable) was fixed at $50,000. |
In connection with the adjustments to the program, the Board, on the recommendation of the Corporate Governance Committee, also increased the stock ownership requirement applicable to non-employee Directors from $300,000 to $550,000 worth of Pfizer stock. For purposes of this requirement, a Director’s holdings include units granted to the Director as compensation for Board service and shares or units held under a deferral or similar plan. A Director has five years from the date of (a) his or her first election as a Director or (b) if later, an increase in the amount of Pfizer stock required to be held, to satisfy this ownership requirement. None of our Directors has pledged Pfizer stock as collateral for personal loans or other obligations.
The changes to the non-employee Director compensation program implemented in 2011 were recommended by the Corporate Governance Committee based upon the advice and recommendations of an independent compensation consulting firm, J.F. Reda & Associates, LLC. The firm was engaged directly by the Committee to provide advice and recommendations on the compensation of non-Employee Directors and does not render any other services to Pfizer.
| 18 |

|
2012 PROXY STATEMENT |
Table of Contents
GOVERNANCE OF THE COMPANY
Under his employer’s policy, Dr. Ausiello is subject to limitations on the amount of compensation he can receive from the Company and is not permitted to receive any equity compensation for serving as a Director. As a result, Dr. Ausiello receives the customary cash fees for his Board and Committee service, but the dollar value of his annual equity award, subject to the limitation on the amount of his compensation under his employer’s policy, is credited to a deferred cash account to be paid (with an interest equivalent) following his termination of service as a Director. At the direction of the Corporate Governance Committee, the dollar value of Dr. Ausiello’s equity award in excess of the limitation has been contributed to charity.
As Non-Executive Chairman of the Board in 2011, in lieu of the above compensation, Mr. Lorch received $550,000, divided equally between cash and equity. The cash portion was paid quarterly, and the equity portion was credited quarterly in the form of stock units valued at the closing price of Pfizer common stock on the last day of each quarter. Mr. Lorch will receive dividend equivalents on these units, and the units and accumulated dividends will be payable in cash or in shares of Pfizer common stock, at his election, at or after his retirement from the Board. Effective January 1, 2012, Mr. Lorch is compensated under the new program described above.
Deferred Compensation
Non-employee Directors may defer all or a part of their annual cash retainers under the Unit Award Plan until they cease to be members of the Board. At a Director’s election, the fees held in the Director’s account may be credited either with Pfizer stock units or with interest at the rate of return of an intermediate treasury index. The rate of return of the intermediate U.S. Treasury index for 2011 was 6.08%. The numbers of Pfizer stock units are calculated by dividing the amount of the deferred fee by the closing price of our common stock on the last business day of the fiscal quarter in which the fee is earned. If fees are deferred as Pfizer stock units, the number of stock units in a Director’s account is increased by crediting additional stock units based on the value of any dividends on the common stock. When a Director ceases to be a member of the Board, the amount attributable to stock units held in his or her account is paid in cash or in Pfizer stock, at the Director’s election. The amount of any cash payment is determined by multiplying the number of Pfizer stock units in the account by the closing price of our common stock on the last business day before the payment date.
Legacy Warner-Lambert Equity Compensation Plan
Under the Warner-Lambert Company 1996 Stock Plan, as a result of our merger with Warner-Lambert, all stock options and restricted stock awards outstanding as of June 19, 2000 became immediately exercisable or vested.
Under this plan, the directors of Warner-Lambert could elect to defer any or all of the compensation they received for their services. These deferred amounts could have been credited to a Warner-Lambert common stock equivalent account (the “Equivalent Account”). The Equivalent Account was credited, as of the day the fees would have been payable, with stock credits equal to the number of shares of Warner-Lambert common stock that could have been purchased with the dollar amount of such deferred fees. The former Warner-Lambert directors who joined our Board after the merger—Messrs. Burt, Gray and Lorch—had deferred compensation and were entitled to Warner-Lambert stock credits in the Equivalent Account under this plan. Dividend equivalents received under this plan are reinvested. Upon the closing of the merger, these Warner-Lambert stock credits were converted into Pfizer stock equivalent units. These units will be payable in Pfizer common stock at various times in accordance with the Director’s election. These units are described in footnote 2 to the table under “Securities Ownership.”
| 2012 PROXY STATEMENT |

|
19 |
Table of Contents
GOVERNANCE OF THE COMPANY
Matching Gift Programs
Our non-employee Directors may participate in Pfizer’s matching gift programs, which are available to all employees. Under these programs, the Pfizer Foundation (Pfizer’s philanthropic affiliate) will match contributions to eligible non-profit organizations, up to a maximum of $15,000 per year; contributions to religious and certain other types of non-profit organizations, as well as to individuals and others in need, are not eligible and are not matched. In addition, the Pfizer Foundation will match contributions made to the United Way Campaign, up to a maximum of $15,000 per year. The matching contributions made by the Pfizer Foundation with respect to our non-employee Directors are included in the 2011 Director Compensation Table below and described in footnote 2 to the Table. As indicated above, these matching contributions do not reflect all of the charitable contributions made by our Directors.
2011 Director Compensation Table
The following table shows 2011 compensation for our non-employee Directors.
| NAME | FEES EARNED OR PAID IN CASH ($) |
EQUITY/ ($) |
ALL
OTHER ($) |
TOTAL ($) | ||||||||||||
| Dr. Ausiello(3) |
194,167 | 66,665 | 260,832 | |||||||||||||
| Dr. Brown |
160,000 | 114,565 | 39,458 | 314,023 | ||||||||||||
| Mr. Burns |
130,000 | 114,565 | 244,565 | |||||||||||||
| Mr. Burt(4) |
26,250 | 45,000 | 71,250 | |||||||||||||
| Mr. Cornwell |
151,667 | 114,565 | 12,544 | 278,776 | ||||||||||||
| Dr. Fergusson |
146,667 | 114,565 | 17,050 | 278,282 | ||||||||||||
| Mr. Gray |
148,333 | 114,565 | 36,875 | 299,773 | ||||||||||||
| Dr. Hobbs |
7,473 | 137,500 | 50,000 | 194,973 | ||||||||||||
| Ms. Horner |
123,333 | 114,565 | 18,700 | 256,598 | ||||||||||||
| Mr. Kilts |
148,333 | 114,565 | 15,000 | 277,898 | ||||||||||||
| Mr. Lorch(5) |
275,000 | 275,000 | 13,500 | 563,500 | ||||||||||||
| Mr. Mascotte |
126,667 | 114,565 | 30,000 | 271,232 | ||||||||||||
| Ms. Nora Johnson |
133,333 | 114,565 | 30,000 | 277,898 | ||||||||||||
| Mr. Sanger |
130,000 | 114,565 | 30,000 | 274,565 | ||||||||||||
| Mr. Steere(4) |
21,250 | 65,000 | 86,250 | |||||||||||||
| Dr. Tessier-Lavigne |
7,473 | 137,500 | 144,973 | |||||||||||||
| (1) | Represents stock units awarded in 2011 to Directors who were re-elected at the 2011 Annual Meeting of Shareholders (other than Dr. Ausiello and Mr. Lorch, as discussed below), the reported value of which was calculated by multiplying the closing market price of our common stock on the grant date (April 28, 2011) by the number of units granted (5,500). In 2011, Mr. Lorch was credited with 13,752 stock units; these units were credited quarterly and were valued at the closing price of Pfizer common stock on the last day of each quarter. Each of Drs. Hobbs and Tessier-Lavigne received 6,744 stock units upon being elected a Director on December 12, 2011 (determined by dividing the value of the award, $137,500, by $20.39, the closing price of the Company’s common stock on the date of their election). At the end of 2011, the aggregate number of stock units (including dividend equivalents) held by each current non-employee Director was as follows: Dr. Ausiello, 21,000; Dr. Brown, 100,739; Mr. Burns, 82,763; Mr. Cornwell, 80,240; Dr. Fergusson, 17,628; Mr. Gray, 105,448; Dr. Hobbs, 6,744; Ms. Horner, 109,774; Mr. Kilts, 59,067; Mr. Lorch, 77,849; Mr. Mascotte, 17,628; Ms. Nora Johnson, 27,000; Mr. Sanger, 44,644; and Dr. Tessier-Lavigne, 6,744. See Note 3. |
| (2) | The amounts in this column represent: (a) charitable contributions made in 2011 under our matching gift programs (see “Matching Gift Programs” above), as follows: Dr. Ausiello, $9,600; Dr. Brown, $25,900; Mr. Burt, $45,000 (consisting of matching contributions made in 2011 in respect of 2010 and 2011 contributions by Mr. Burt); Dr. Fergusson, $17,050; Mr. Gray, $20,000; Ms. Horner, $3,700; Mr. Kilts, $15,000; Mr. Lorch, $13,500; Mr. Mascotte, $30,000; Ms. Nora Johnson, $30,000; Mr. Sanger, $30,000; and Mr. Steere, $15,000; (b) charitable contributions totaling $57,065 made at the discretion of the Corporate Governance Committee in respect of Dr. Ausiello (see Note 3 below); (c) for Dr. Brown, $13,558 for travel and related activities associated with attendance by Dr. Brown’s spouse at an offsite meeting of the Board and other events to encourage attendance and foster interaction among members of the Board and management; (d) for Mr. Cornwell, $12,544 for travel and related activities associated with attendance by Mr. Cornwell’s spouse at an offsite meeting of the Board and other events to encourage attendance and foster interaction among members of the Board and management; (e) for Mr. Gray, (i) above-market interest on the deferred cash balance under a legacy Warner-Lambert equity compensation plan, paid at the prime rate plus 2%, and (ii) attendance by Mr. Gray’s spouse at an off-site meeting of the Board of Directors to encourage attendance and foster interaction among the members of the Board and management; (f) for Dr. Hobbs, $50,000 for serving on a Pfizer Scientific Advisory Panel for a therapeutic area of research, which service terminated prior to her election as a Director in December 2011; (g) for Ms. Horner, a $15,000 charitable contribution made in honor of her service to the Board and the Company during her tenure as Lead Independent Director until December 2010; and (h) for Mr. Steere, $50,000 relating to his consulting contract with the Company (see “Section 16(a) Beneficial Ownership Reporting Compliance, Related Person Transactions and Indemnification—Transactions with Related Persons”). As indicated above under “Matching Gift Programs,” certain charitable contributions by our Directors are not eligible for matching contributions under the programs, and the amounts in the above table therefore do not reflect all such contributions made by our Directors. |
| (3) | Dr. Ausiello’s employer limits the amount of compensation he can receive from the Company and prohibits him from receiving any equity compensation for serving as a Director. For 2011, he received $136,667 in cash compensation, and an additional $57,500 was credited to a deferred cash account to be paid (with an interest equivalent) following his termination of service as a Director. See “Non-Employee Director Compensation” and Note 2 above. |
| (4) | Messrs. Burt and Steere retired as Directors at the 2011 Annual Meeting of Shareholders. |
| (5) | Mr. Lorch served as Non-Executive Chairman of the Board until December 2011. |
| 20 |

|
2012 PROXY STATEMENT |
Table of Contents
The table below shows the number of shares of our common stock beneficially owned as of the close of business on January 31, 2012 by each of our Directors and each Named Executive Officer listed in the 2011 Summary Compensation Table, as well as the number of shares beneficially owned by all of our Directors and executive officers as a group. Together, these individuals beneficially own less than one percent (1%) of our common stock outstanding. The table and footnotes also include information about stock options, stock appreciation rights in the form of total shareholder return units (“TSRUs”), stock units, restricted stock, restricted stock units and deferred performance-related share awards credited to the accounts of our Directors and executive officers under various compensation and benefit plans.
| BENEFICIAL OWNERS |
NUMBER OF SHARES OR UNITS | OPTIONS EXERCISABLE WITHIN 60 DAYS |
||||||||||
| COMMON STOCK | STOCK UNITS |
|||||||||||
| Dennis A. Ausiello |
2,362 | (1) | 21,000 | (2) | ||||||||
| Michael S. Brown |
1,200 | 100,739 | (2) | |||||||||
| M. Anthony Burns |
24,978 | 82,763 | (2) | |||||||||
| W. Don Cornwell |
2,000 | (1) | 80,240 | (2) | ||||||||
| Frank A. D’Amelio |
181,843 | (3) | 343,564 | (4) | 292,000 | |||||||
| Mikael Dolsten |
190 | (3) | 218,948 | (4) | ||||||||
| Frances D. Fergusson |
17,628 | (2) | ||||||||||
| William H. Gray, III |
28 | 105,448 | (2) | |||||||||
| Helen H. Hobbs |
6,744 | (2) | ||||||||||
| Constance J. Horner |
15,825 | 109,774 | (2) | |||||||||
| James M. Kilts |
2,259 | (1) | 59,067 | (2) | ||||||||
| George A. Lorch |
24,126 | 77,849 | (2) | |||||||||
| John P. Mascotte |
3,940 | 17,628 | (2) | |||||||||
| Suzanne Nora Johnson |
10,000 | 27,000 | (2) | |||||||||
| Ian C. Read |
377,582 | (3) | 512,463 | (4) | 873,000 | |||||||
| Stephen W. Sanger |
1,085 | (1) | 44,644 | (2) | ||||||||
| Amy W. Schulman |
61,748 | (1)(3) | 133,787 | (4) | 100,000 | |||||||
| David Simmons |
40,124 | (3) | 113,704 | (4) | 124,500 | |||||||
| Marc Tessier-Lavigne |
104 | 6,744 | (2) | |||||||||
| All Directors and Executive Officers as a group (27) |
991,326 | 2,990,481 | 2,128,400 | |||||||||
| (1) | Includes the following shares held in the names of family members: Dr. Ausiello, 2,362 shares; Mr. Cornwell, 300 shares; Mr. Kilts, 2,259 shares; Mr. Sanger, 1,085 shares; and Ms. Schulman, 300 shares. Dr. Ausiello, Ms. Schulman and Messrs. Cornwell and Kilts disclaim beneficial ownership of such shares. |
| (2) | Represents units (each equivalent to a share of Pfizer common stock) awarded under our Director compensation plans (see “Compensation of Non-Employee Directors” above). This number also includes the following units resulting from the conversion into Pfizer units of previously deferred Warner-Lambert director compensation under the Warner-Lambert 1996 Stock Plan: Mr. Gray, 57,819 units and Mr. Lorch, 15,219 units. See “Compensation of Non-Employee Directors—Legacy Warner-Lambert Equity Compensation Plan” above. |
| (3) | Includes shares credited under the Pfizer Savings Plan and/or deferred performance shares relating to previously vested awards under the Company’s performance-based share award programs. These plans are described later in this Proxy Statement. |
| (4) | In the case of Messrs. D’Amelio, Read and Simmons and Ms. Schulman, includes units (each equivalent to a share of Pfizer common stock) held under the Pfizer Supplemental Savings Plan and for Mr. Simmons also includes units held under the Pfizer Inc. Deferred Compensation Plan. The Pfizer Supplemental Savings Plan is described later in this Proxy Statement. Also includes the following unvested restricted stock units (each equivalent to a share of Pfizer common stock): Mr. D’Amelio, 322,768; Dr. Dolsten, 218,948; Mr. Read, 406,900 (however, in view of Mr. Read’s age and years of service with Pfizer, a prorated portion of his units would vest upon his retirement); Mr. Simmons, 98,874; and Ms. Schulman, 130,209. This column does not include the following stock appreciation rights in the form of TSRUs: Mr. D’Amelio, 1,039,555; Dr. Dolsten, 530,757; Mr. Read, 2,131,948; Ms. Schulman, 516,701; and Mr. Simmons, 467,124. See the “2011 Outstanding Equity Awards at Fiscal Year-End Table” and “Estimated Benefits upon Termination” for a discussion of the vesting of restricted stock units and TSRUs. |
Beneficial Owners
Based on filings made under Sections 13(d) and 13(g) of the Securities Exchange Act of 1934, as amended, as of December 30, 2011, the only person known by us to be the beneficial owner of more than 5% of our common stock was as follows:
| NAME AND ADDRESS OF BENEFICIAL OWNER(1) | SHARES OF PFIZER COMMON STOCK(1) |
PERCENT OF CLASS | ||||||
| BlackRock, Inc. 40 East 52nd Street New York, NY 10022 |
463,841,882 | 6.03% | ||||||
| (1) | This information is based solely on a Schedule 13G filed with the SEC on February 13, 2012 by BlackRock, Inc. |
| 2012 PROXY STATEMENT |

|
21 |
Table of Contents
Section 16(a) Beneficial Ownership Reporting Compliance, Related Person Transactions, and Indemnification
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our Directors and certain of our officers to file reports of holdings and transactions in Pfizer equity with the SEC and the NYSE. Based on our records and other information, we believe that in 2011 our Directors and our officers who are subject to Section 16(a) met all applicable filing requirements. There were two exceptions:
| • | Upon becoming subject to Section 16 in December 2010, Olivier Brandicourt, President and General Manager of our Primary Care Business Unit, filed a Form 3 with the SEC on a timely basis. Due to an inadvertent administrative error by the Company’s external plan administrator, the Form 3 included an incorrect number of shares of Pfizer common stock (5,494) held in the Company’s Savings Plan. Promptly after being informed of the error, Dr. Brandicourt filed an amendment to the Form 3 reporting the correct number of shares (8,965) held in the Plan. |
| • | Upon his election to the Board of Directors in December 2011, Dr. Tessier-Lavigne filed a Form 3 with the SEC on a timely basis that inadvertently failed to include 104 shares of Pfizer common stock held in a brokerage account that is managed by a portfolio manager. Promptly after being informed of the omission, Dr. Tessier-Lavigne filed an amendment to the Form 3 reporting the ownership of those shares. |
REVIEW OF RELATED PERSON TRANSACTIONS
The Company has adopted a Related Person Transaction Approval Policy that is administered by the Corporate Governance Committee. The Policy applies to any transaction or series of transactions in which the Company or a subsidiary is a participant, the amount involved exceeds $120,000, and a related person has a direct or indirect material interest. Under the Policy, Company management determines whether a transaction requires review by the Committee, and transactions requiring review are referred to the Committee for approval, ratification or other action. Based on its consideration of all of the relevant facts and circumstances, the Committee decides whether or not to approve such transactions and approves only those transactions that are deemed to be in the best interests of the Company. If the Company becomes aware of an existing transaction with a related person that has not been approved under this Policy, the matter is referred to the Committee. The Committee then evaluates all options available, including ratification, revision or termination of such transaction.
TRANSACTIONS WITH RELATED PERSONS
In connection with his retirement in 2001, we entered into a consulting agreement with Mr. Steere, a member of our Board of Directors until April 2011. The agreement provides that Mr. Steere would serve as Chairman Emeritus of the Company and, when and as requested by the Chief Executive Officer, will provide consulting services and advice to the Company and participate in various external activities and events for the benefit of the Company. The term of the agreement, which began on July 1, 2001 after Mr. Steere ceased his employment with the Company, was for five years, with automatic extensions for successive five-year terms, unless Mr. Steere or the Company terminates the agreement at the end of its then-current term. The contract was extended for a five-year term in 2011 and currently extends until mid-2016. Mr. Steere may provide up to 30 days of service per year to the Company, subject to his reasonable availability, for his consulting services or his participation as a Company representative in external activities and events. He must obtain the approval of the Board of Directors before providing any consulting services, advice or service of any kind to any other company or organization that competes with us. For his services and commitments, the Company pays Mr. Steere (i) an annual retainer of $50,000 for his consulting services (subject to his ability to continue to provide the contemplated services), and (ii) an additional fee of $5,000 for each day in excess of 30 days per year that he renders services as described above. We also reimburse him for reasonable expenses that he incurs in providing these services for us.
In addition, under the terms of the agreement, we provide him lifetime access to Company facilities and services comparable to those that were made available to him by the Company prior to his retirement. These include the use of an office and access to the secretarial services of an administrative assistant; access to financial planning services; and the use of a car and driver and of Company aircraft. Mr. Steere has chosen to personally pay for his financial planning services and voluntarily reimburses the Company for all personal use of Company-provided transportation.
We paid Mr. Steere $50,000 in 2011 under the terms of this consulting agreement.
We indemnify our Directors and our elected officers to the fullest extent permitted by law so that they will be free from undue concern about personal liability in connection with their service to the Company. This is required under our By-laws, and we have also entered into agreements with those individuals contractually obligating us to provide this indemnification to them.
| 22 |

|
2012 PROXY STATEMENT |
Table of Contents
| 2012 PROXY STATEMENT |

|
23 |
Table of Contents
|
DENNIS A. AUSIELLO, 66
Position, Principal Occupation and Business Experience:
Jackson Professor of Clinical Medicine at Harvard Medical School and Chief of Medicine at Massachusetts General Hospital since 1996. President of the Association of American Physicians in 2006. Member of the Institute of Medicine of the National Academies of Science and a Fellow of the American Academy of Arts and Sciences. Director of TARIS BioMedical, Inc. and of several non-profit organizations, including the Broad Institute for Human Genetics.
|
| |||||
| Key Attributes, Experience and Skills:
Dr. Ausiello’s experience and training as a practicing physician (Board certified in nephrology), a scientist and a nationally recognized leader in academic medicine enable him to bring valuable insights to the Board, including through his understanding of the scientific nature of our business and the ability to assist us in prioritizing opportunities for drug development. In addition, Dr. Ausiello oversees a large research portfolio and an extensive research and education budget at Massachusetts General Hospital, giving him a critical perspective on drug discovery and development and providing a fundamental understanding of the potential pathways contributing to disease. Through his work as the Chief of Medicine at Massachusetts General Hospital, Dr. Ausiello also brings leadership, oversight and finance experience to the Board. |
Director Since: 2006
Board Committees: Audit, Corporate Governance, Regulatory and Compliance, and Science and Technology
|
|||||
|
M. ANTHONY BURNS, 69
Position, Principal Occupation and Business Experience:
Chairman Emeritus since 2002, Chairman of the Board from 1985 to 2002, Chief Executive Officer from 1983 to 2000, and President from 1979 to 1999 of Ryder System, Inc., a provider of transportation and logistics services. Life Trustee of the University of Miami. Director of J. C. Penney Company, Inc. from 1988 to May 2011; Stanley Black & Decker, Inc. from March 2010 until May 2010; and The Black & Decker Corporation from 2001 until March 2010.
|
| |||||
| Key Attributes, Experience and Skills:
As a result of Mr. Burns’ long tenure as CEO of Ryder System, he provides valuable business, leadership and management insights into driving strategic direction and international operations, among other things. While at Ryder, Mr. Burns was responsible for Ryder’s expansion into international markets, which is important as Pfizer seeks to execute its global growth strategies. In addition, Mr. Burns brings financial expertise to the Board, including through his service on (and in some cases chairmanship of) the audit committees of other public companies, as well as executive compensation experience, including through his service on the compensation committees of several public companies, including prior service on our Compensation Committee. Mr. Burns also served as co-chairman of the Business Roundtable from 1998 to 2001, providing him with exposure to and insight from, CEOs of other large companies. |
Director Since: 1988
Board Committees: Audit and Corporate Governance
Directorship:
|
|||||
| 24 |

|
2012 PROXY STATEMENT |
Table of Contents
NOMINEES FOR DIRECTORS
|
W. DON CORNWELL, 64
Position, Principal Occupation and Business Experience:
Chairman of the Board and Chief Executive Officer of Granite Broadcasting Corporation from 1988 until his retirement in August 2009 and Vice Chairman until December 2009. Granite Broadcasting Corporation filed for voluntary reorganization under Chapter 11 of the U.S. Bankruptcy Code in December 2006 and emerged from its restructuring in June 2007. Director of the Wallace Foundation and Trustee of Big Brothers/Sisters of New York and the M.S. Hershey School and Trust from 1995 until 2002.
Key Attributes, Experience and Skills:
Through Mr. Cornwell’s 38-year career as an entrepreneur driving the growth of a consumer-focused media company, an executive in the investment banking industry and a director of several significant consumer product and healthcare companies, he has valuable business, leadership and management experience and brings important perspectives on the issues facing our Company. Mr. Cornwell founded and built Granite, a consumer-focused media company, through acquisitions and operating growth, enabling him to provide insight and guidance on strategic direction and growth. Mr. Cornwell’s strong financial background, including his work at Goldman Sachs prior to co-founding Granite and his service on the audit and investment committees of other companies, also provides financial expertise to the Board, including an understanding of financial statements, corporate finance, accounting and capital markets. |
| |||||
|
Director Since: 1997
Board Committees: Audit (Chair), Compensation, and Regulatory and Compliance
Directorships:
|
||||||
|
FRANCES D. FERGUSSON, 67
Position, Principal Occupation and Business Experience:
President Emeritus of Vassar College since 2006 and President from 1986 to 2006. Served on the Mayo Clinic Board for 14 years, the last four years as its Chairman, and as President of the Board of Overseers of Harvard University from 2007 through 2008. Director of HSBC Bank USA from 1990 through 2008 and of Wyeth from 2005 until 2009.
Key Attributes, Experience and Skills:
Dr. Fergusson has strong leadership skills, having served as President of Vassar College for 20 years and, during her tenure, developing a long-term financial plan and strengthening the College’s financial position. She has also headed strategic planning projects at Vassar and other organizations. Dr. Fergusson’s service on the boards of not-for-profit organizations, including the Mayo Clinic (which she chaired from 1988 to 2002), enables her to bring to the Board experience and knowledge of healthcare from alternate perspectives. In addition, Dr. Fergusson’s past service on the Wyeth Board of Directors affords her extensive knowledge of Wyeth’s business, operations and culture, which brings a connection to that portion of our business and operations. |
| |||||
|
Director Since: 2009
Board Committees: Regulatory and Compliance (Chair), Compensation, and Science and Technology
Directorship:
|
||||||
| 2012 PROXY STATEMENT |

|
25 |
Table of Contents
NOMINEES FOR DIRECTORS
|
WILLIAM H. GRAY, III , 70
Position, Principal Occupation and Business Experience:
Chairman of Gray Global Strategies, Inc., a business advisory firm. Co-Chairman of GrayLoeffler, LLC from 2009-2011, a business advisory and consulting firm. Chairman of the Amani Group, its predecessor, from 2004 through September 2009. Pastor Emeritus of the Bright Hope Baptist Church in Philadelphia since 2005. President and Chief Executive Officer of The College Fund/UNCF (Educational Assistance) from 1991 to 2004. U.S. Congressman from the Second District of Pennsylvania from 1979 to 1991, including service at various times as Budget Committee Chair and House Majority Whip. Director of Visteon Corporation from 2000 until January 2010.
Key Attributes, Experience and Skills:
Mr. Gray’s experience as a U.S. Congressman for 12 years, including his service as Budget Committee Chair and House Majority Whip, position him to provide advice and counsel to our Company in a highly regulated industry and to provide guidance in government relations. Mr. Gray also has valuable experience running a national organization on financial literacy and macro-economic policy. Mr. Gray also brings useful corporate governance and compliance insights from, among other things, his role as an Advisory Council Member of the Business Roundtable Institute for Corporate Ethics. |
| |||||
|
Director Since: 2000
Board Committees: Corporate Governance (Chair) and Science and Technology
Directorships: Dell Inc., J. P. Morgan Chase & Co. and Prudential Financial, Inc.
|
||||||
|
HELEN H. HOBBS, 59
Position, Principal Occupation and Business Experience:
Investigator of the Howard Hughes Medical Institute since 2002, a Professor of Internal Medicine and Molecular Genetics and Director of the McDermott Center for Human Growth and Development at the University of Texas Southwestern Medical Center. In 2007, Dr. Hobbs was elected to the National Academy of Sciences and received the Distinguished Scientist Award from the American Heart Association. In 2005, she became the first recipient of the Clinical Scientist Award from the American Heart Association, and was awarded Germany’s Heinrich Wieland Prize. Dr. Hobbs was elected to the Institute of Medicine in 2004 and the American Academy of Arts and Sciences in 2006. She is a member of the American Society of Clinical Investigation and the Association of American Physicians.
Key Attributes, Experience and Skills:
Dr. Hobbs’s background reflects great achievements in academia and medicine. She has served as a faculty member at the University of Texas Southwestern Medical Center for more than 20 years, and is a leading geneticist in the arena of metabolism and heart disease, areas in which Pfizer has significant investments and experience. Pfizer expects to benefit from her experience, expertise and achievements in both medicine and science. |
| |||||
|
Director Since: 2011
Board Committees: Corporate Governance and Science and Technology
|
||||||
| 26 |

|
2012 PROXY STATEMENT |
Table of Contents
NOMINEES FOR DIRECTORS
|
CONSTANCE J. HORNER, 70
Position, Principal Occupation and Business Experience:
Guest Scholar from 1993 until 2005 at The Brookings Institution, an organization devoted to nonpartisan research, education and publication in economics, government, foreign policy and the social sciences. Commissioner of the U.S. Commission on Human Rights from 1993 to 1998. Served at the White House as Assistant to President George H. W. Bush and as Director of Presidential Personnel from 1991 to 1993. Deputy Secretary, U.S. Department of Health and Human Services, from 1989 to 1991. Director of the U.S. Office of Personnel Management from 1985 to 1989. Fellow, National Academy of Public Administration, and Member of the Board of Trustees of the Prudential Foundation.
Key Attributes, Experience and Skills:
Ms. Horner is well-versed in federal health and health financing policy as well as talent management as a result of her service as the head of the U.S. Office of Personnel Management, which, among other responsibilities, designs and administers the health insurance program for federal employees and retirees and manages policies and programs for the recruitment, training and compensation of the federal workforce; her chairmanship of a White House Competitiveness Council task force making recommendations to improve the drug approval process; and her service as Deputy Secretary of the U.S. Department of Health and Human Services, where she had responsibility for the Food and Drug Administration, the National Institutes of Health, the Public Health Service and the Health Care Financing Administration (now the Center for Medicare and Medicaid Services), lending insight into how the federal government makes health policies that affect Pfizer’s ability to create products and get them to the people who need them. In addition, Ms. Horner’s government experience positions her to provide oversight to our Company in government relations, including regulatory areas. |
| |||||
|
Director Since: 1993
Board Committees: Corporate Governance and Regulatory and Compliance
Directorships: Ingersoll-Rand plc and Prudential Financial, Inc.
|
||||||
|
JAMES M. KILTS, 64
Position, Principal Occupation and Business Experience:
Founding Partner, Centerview Capital, a private equity firm, since 2006. Vice Chairman, The Procter & Gamble Company, from 2005 to 2006. Chairman and Chief Executive Officer, The Gillette Company, from 2001 to 2005 and President, The Gillette Company, from 2003 to 2005. President and Chief Executive Officer, Nabisco Group Holdings Corporation, from 1998 until its acquisition in 2000. Currently Chairman of The Nielsen Company B.V. Supervisory Board and Non-Executive Chairman of the Board of Nielsen Holdings N.V. Trustee of Knox College and the University of Chicago, and a member of the Board of Overseers of Weill Cornell Medical College. Director of New York Times Company from 2005 until 2008.
Key Attributes, Experience and Skills:
Mr. Kilts’ tenure as CEO of Gillette and Nabisco and as Vice Chairman of Procter & Gamble provides valuable business, leadership and management experience, including expertise in cost management, creating value and resource allocation. In addition, Mr. Kilts’ knowledge of consumer businesses has given him insights on reaching consumers and on the importance of innovation—both important aspects of Pfizer’s business. Through his service on the board of MetLife, an insurance company, Mr. Kilts can offer a view of healthcare from another perspective, and through Mr. Kilts’ service on three compensation committees, including ours, he has a strong understanding of executive compensation and related areas. |
| |||||
|
Director Since: 2007
Board Committees: Compensation (Chair) and Science and Technology
Directorships: Meadwestvaco Corporation, MetLife, Inc. and Nielsen Holdings N.V.
|
||||||
| 2012 PROXY STATEMENT |

|
27 |
Table of Contents
NOMINEES FOR DIRECTORS
|
GEORGE A. LORCH, 70
Position, Principal Occupation and Business Experience:
Chairman Emeritus of Armstrong Holdings, Inc., a global manufacturer of flooring and ceiling materials, since 2000, having served as Chairman and Chief Executive Officer and in other executive capacities with Armstrong Holdings, Inc. and its predecessor, Armstrong World Industries, Inc., from 1993 to 2000. Director of Masonite International, Inc., a non-public company, and also a Director of HSBC Finance Co. and HSBC North America Holding Company, non-public, wholly owned subsidiaries of HSBC LLC. Director of The Williams Companies, Inc. until December 2011.
Key Attributes, Experience and Skills:
Mr. Lorch’s service as CEO of Armstrong Holdings provides valuable business, leadership and management experience, including expertise leading a large organization with global operations, giving him a keen understanding of the issues facing a multinational business such as Pfizer’s. In addition, Mr. Lorch has significant experience with manufacturing, marketing and branding, all important areas for Pfizer. Mr. Lorch’s experience on the board of directors of Autoliv, a non-U.S.-based public company, enables him to bring global perspectives and experience to the Board, including best practices gained from other countries. Moreover, his service on three compensation committees (including ours, until December 2010) has given him a strong understanding of executive compensation and related areas. |
| |||||
|
Director Since: 2000
Lead Independent Director
Directorships: Autoliv, Inc. and WPX Energy, Inc.
|
||||||
|
JOHN P. MASCOTTE, 72
Position, Principal Occupation and Business Experience:
Retired President and Chief Executive Officer of Blue Cross and Blue Shield of Kansas City, Inc., a position he held from 1997 through 2001. Former Chairman of Johnson & Higgins of Missouri, Inc. and former Chairman and Chief Executive Officer of The Continental Corporation. Served on the boards of The New York Public Library, Lincoln Center and The Aspen Institute and as Chairman of The Local Initiative Support Corporation, The Aspen Community Foundation and Common Cents. Director of Wyeth from 1995 until 2009.
Key Attributes, Experience and Skills:
Mr. Mascotte’s service as CEO of Blue Cross and Blue Shield of Kansas City, Inc., a healthcare insurance company, and as Chairman and CEO of The Continental Corporation, an insurance holding company, for 12 years, provides him with valuable business, leadership and management experience, and enables him to lend insight on an insurance company’s perspective of the biopharmaceutical industry. In addition, Mr. Mascotte has significant knowledge of Wyeth’s business, operations and culture as a result of his 14 years of service on the Wyeth Board of Directors, which brings a connection to that portion of our business and operations. Mr. Mascotte also brings financial expertise to the Board through his chairmanship of the Audit Committee of Wyeth and his prior work as a certified public accountant and tax specialist. |
| |||||
|
Director Since: 2009
Board Committees: Corporate Governance, Regulatory and Compliance, and Science and Technology
|
||||||
| 28 |

|
2012 PROXY STATEMENT |
Table of Contents
NOMINEES FOR DIRECTORS
|
SUZANNE NORA JOHNSON, 54
Position, Principal Occupation and Business Experience:
Retired Vice Chairman, Goldman Sachs Group, Inc., since 2007. During her 21-year tenure with Goldman Sachs, she served in various leadership roles, including Chair of the Global Markets Institute, Head of Global Research, and Head of Global Healthcare. Board member of the American Red Cross, The Brookings Institution, the Carnegie Institution of Washington and the University of Southern California.
Key Attributes, Experience and Skills:
Ms. Nora Johnson’s careers in law and investment banking, including serving in various leadership roles at Goldman Sachs, provide valuable business experience and critical insights on the roles of the law, finance and strategic transactions to our business. In addition, Ms. Nora Johnson’s extensive knowledge of healthcare through her role in healthcare investment banking and her involvement with not-for-profit organizations, such as in scientific research (The Carnegie Institution), healthcare policy (RAND Corporation and The Brookings Institution), and healthcare services (the American Red Cross), provide touchstones of public opinion and exposure to diverse, global points of view. Ms. Nora Johnson also brings financial expertise to the Board, providing an understanding of financial statements, corporate finance, accounting and capital markets. |
| |||||
|
Director Since: 2007
Board Committees: Audit, Compensation, and Science and Technology
Directorships: American International Group, Inc., Intuit Inc. and VISA Inc.
|
||||||
|
IAN C. READ, 58
Position, Principal Occupation and Business Experience:
Chairman and Chief Executive Officer since December 2011. President and Chief Executive Officer from December 2010 until December 2011. Senior Vice President, Group President of the Worldwide Biopharmaceutical Businesses (Primary Care, Specialty Care, Oncology, Established Products and Emerging Markets) from 2006 through December 2010. Since joining Pfizer in 1978 as an operational auditor, Mr. Read has held various positions of increasing responsibility in pharmaceutical operations. He worked in Latin America through 1995, holding positions including Chief Financial Officer, Pfizer Mexico, and Country Manager, Pfizer Brazil. In 1996, Mr. Read was appointed President of Pfizer’s International Pharmaceuticals Group, with responsibility for Latin America and Canada. He became Executive Vice President, Europe in 2000, was named a Corporate Vice President in 2001, and assumed responsibility for Canada, in addition to Europe, in 2002. Mr. Read later became accountable for operations in both the Africa/Middle East region and Latin America as well. Serves on the Boards of Pharmaceutical Research and Manufacturers of America (PhRMA), the European Federation of Pharmaceutical Industries and Associations, and the Partnership for New York City. Member of our Executive Leadership Team
Key Attributes, Experience and Skills:
Mr. Read brings over 30 years of business, operating and leadership experience to the Board. His extensive knowledge of the biopharmaceutical industry in general, and Pfizer’s worldwide biopharmaceutical business in particular, provides crucial insight to our Board on the Company’s strategic planning and operations. Mr. Read provides an essential link between management and the Board on management’s business perspectives, and the combination of his knowledge of the business and his leadership skills make his role as Chairman and CEO optimal at this time. Further, his experience as a member of another public company board provides him with an enhanced perspective on issues applicable to public companies. |
| |||||
|
Director Since: 2010
Directorship: Kimberly-Clark Corporation
|
||||||
| 2012 PROXY STATEMENT |

|
29 |
Table of Contents
NOMINEES FOR DIRECTORS
|
STEPHEN W. SANGER, 66
Position, Principal Occupation and Business Experience:
Chairman of General Mills, Inc., a packaged food producer and distributor, from 1995 until his retirement in 2008 and its Chief Executive Officer from 1995 to 2007. Former Chairman of the Grocery Manufacturers of America. Recipient of the Woodrow Wilson Award for Public Service in 2009. Chaired the Fiscal Policy Committee of the Business Roundtable and served as a director of Catalyst. Director of General Mills, Inc. from 1992 until 2008.
Key Attributes, Experience and Skills:
With more than 12 years’ experience as Chairman and CEO of General Mills, Mr. Sanger has valuable business, leadership and management experience, including experience in acquisitions through the purchase of Pillsbury, creating one of the world’s largest food companies. As CEO of General Mills, Mr. Sanger improved sales and market position, developed innovative ideas and streamlined operations, skills from which Pfizer may benefit. In addition, Mr. Sanger has experience leading a company whose products are subject to FDA regulation, lending insight into the regulated nature of our business. |
| |||||
|
Director Since: 2009
Board Committees: Audit and Corporate Governance
Directorships: Target Corporation and Wells Fargo & Company
|
||||||
|
MARC TESSIER-LAVIGNE, 52
Position, Principal Occupation and Business Experience:
President of The Rockefeller University since March 2011. Between 2003 and 2011, held positions of increasing responsibility at Genentech, where he became Executive Vice President, Research, and Chief Scientific Officer. Susan B. Ford Professor in the School of Humanities and Sciences, and Professor of Biological Sciences and of Neurology and Neurological Sciences, at Stanford University from 2001 to 2003, and a faculty member at the University of California, San Francisco from 1991 to 2001. In addition, Dr. Tessier-Lavigne was a Howard Hughes Medical Institute Investigator from 1994 to 2003. Member of the National Academy of Sciences and its Institute of Medicine, and a Fellow of the Royal Society (UK), the Royal Society of Canada, the Academy of Medical Sciences (UK) and the American Association for the Advancement of Science.
Key Attributes, Experience and Skills:
Dr. Tessier-Lavigne’s background reflects great achievements in a wide variety of disciplines. His business experience includes a senior management role at Genentech, demonstrating his understanding of the role of science in business; his achievements and credentials in science and medicine reflect significant medical and scientific knowledge; and his previous and current roles in academia provide an understanding of the role of research in the pharmaceutical industry. Pfizer expects to benefit from his experience and expertise in these and other areas. |
| |||||
|
Director Since: 2011
Board Committees: Regulatory and Compliance and Science and Technology
Directorship: Regeneron
|
||||||
| 30 |

|
2012 PROXY STATEMENT |
Table of Contents
RATIFICATION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
| 2012 PROXY STATEMENT |

|
31 |
Table of Contents
RATIFICATION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
2. Audit-related services are for assurance and related services that are traditionally performed by the independent registered public accounting firm, including due diligence related to mergers and acquisitions, employee benefit plan audits, and special procedures required to meet certain regulatory requirements.
3. Tax services include all services, except those services specifically related to the audit of the financial statements, performed by the independent registered public accounting firm’s tax personnel, including tax analysis; assisting with coordination of execution of tax-related activities, primarily in the area of corporate development; supporting other tax-related regulatory requirements; and tax compliance and reporting.
4. All other services are those services not captured in the audit, audit-related or tax categories. The Company generally does not request such services from the independent registered public accounting firm.
Prior to engagement, the Audit Committee pre-approves independent registered public accounting firm services within each category, and the fees for each category are budgeted. The Audit Committee requires the independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service. During the year, circumstances may arise when it may become necessary to engage the independent registered public accounting firm for additional services not contemplated in the original pre-approval categories. In those instances, the Audit Committee requires specific pre-approval before engaging the independent registered public accounting firm.
The Audit Committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report, for informational purposes only, any pre-approval decisions to the Audit Committee at its next scheduled meeting.
The Audit Committee reviews the Company’s financial reporting process on behalf of the Board of Directors. Management has the primary responsibility for the financial statements and the reporting process, including the system of internal controls.
In this context, the Committee has met and held discussions with management and the independent registered public accounting firm regarding the fair and complete presentation of the Company’s results and the assessment of the Company’s internal control over financial reporting. The Committee has discussed significant accounting policies applied by the Company in its financial statements, as well as alternative treatments. Management has represented to the Committee that the Company’s consolidated financial statements were prepared in accordance with accounting principles generally accepted in the United States of America, and the Committee has reviewed and discussed the consolidated financial statements with management and the independent registered public accounting firm. The Committee has discussed with the independent registered public accounting firm matters required to be discussed by Statement on Auditing Standards No. 114.
In addition, the Committee has reviewed and discussed with the independent registered public accounting firm the auditor’s independence from the Company and its management. As part of that review, the Committee has received the written disclosures and the letter required by applicable requirements of the Public Company Accounting Oversight Board regarding the independent accountant’s communications with the Audit Committee concerning independence, and the Committee has discussed the independent registered public accounting firm’s independence from the Company.
The Committee also has considered whether the independent registered public accounting firm’s provision of non-audit services to the Company is compatible with the auditor’s independence. The Committee has concluded that the independent registered public accounting firm is independent from the Company and its management.
As part of its responsibilities for oversight of the Company’s Enterprise Risk Management process, the Committee has reviewed and discussed Company policies with respect to risk assessment and risk management, including discussions of individual risk areas, as well as an annual summary of the overall process.
The Committee has discussed with the Company’s Internal Audit Department and independent registered public accounting firm the overall scope of and plans for their respective audits. The Committee meets with the Chief Internal Auditor, Chief Compliance and Risk Officer, and representatives of the independent registered public accounting firm, in regular and executive sessions, to discuss the results of their examinations, the evaluations of the Company’s internal controls, and the overall quality of the Company’s financial reporting and compliance programs.
| 32 |

|
2012 PROXY STATEMENT |
Table of Contents
AUDIT COMMITTEE REPORT
In reliance on the reviews and discussions referred to above, the Committee has recommended to the Board of Directors, and the Board has approved, that the audited financial statements be included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2011, for filing with the SEC. The Committee has selected, and the Board of Directors has ratified, the selection of the Company’s independent registered public accounting firm for 2012.
The Audit Committee
The Audit Committee Report does not constitute soliciting material, and shall not be deemed to be filed or incorporated by reference into any other Company filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Company specifically incorporates the Audit Committee Report by reference therein.
| 2012 PROXY STATEMENT |

|
33 |
Table of Contents
ADVISORY APPROVAL OF EXECUTIVE COMPENSATION
| 34 |

|
2012 PROXY STATEMENT |
Table of Contents
ADVISORY APPROVAL OF EXECUTIVE COMPENSATION
| 2012 PROXY STATEMENT |

|
35 |
Table of Contents
We expect the following proposals (Items 4 through 7 on the proxy card) to be presented by shareholders at the Annual Meeting. Some of the proposals contain assertions about Pfizer or other statements that we believe are incorrect. We have not attempted to refute all these inaccuracies. However, the Board of Directors has recommended a vote against these proposals for the broader policy reasons set forth following each proposal. The names, addresses and share holdings of any co-filers of these proposals, where applicable, will be supplied upon request.
ITEM 4 – SHAREHOLDER PROPOSAL REGARDING PUBLICATION OF POLITICAL CONTRIBUTIONS
Mrs. Evelyn Y. Davis, Watergate Office Building, 2600 Virginia Avenue, N.W., Suite 215, Washington, DC 20037, who represents that she owns 1,200 shares of Pfizer common stock, has submitted the following proposal for consideration at the Annual Meeting:
RESOLVED: “That the stockholders recommend that the Board direct management that within five days after approval by the shareholders of this proposal, the management shall publish in newspapers of general circulation in the cities of New York, Washington, D.C., Detroit, Chicago, San Francisco, Los Angeles, Dallas, Houston and Miami, and in the Wall Street Journal and U.S.A. Today, a detailed statement of each contribution made by the Company, either directly or indirectly, within the immediately preceding fiscal year, in respect of a political campaign, political party, referendum or citizens’ initiative, or attempts to influence legislation, specifying the date and amount of each such contribution, and the person or organization to whom the contribution was made. Subsequent to this initial disclosure, the management shall cause like data to be included in each succeeding report to shareholders.” “And if no such disbursements were made, to have that fact publicized in the same manner.”
REASONS: “This proposal, if adopted, would require the management to advise the shareholders how many corporate dollars are being spent for political purposes and to specify what political causes the management seeks to promote with those funds. It is therefore no more than a requirement that the shareholders be given a more detailed accounting of these special purpose expenditures that they now receive. These political contributions are made with dollars that belong to the shareholders as a group and they are entitled to know how they are being spent.”
“Last year the owners of shares representing 4.6% of the votes cast voted FOR this proposal.”
“If you AGREE, please mark your proxy FOR this resolution.”
| Your Company’s Response: |
The Board of Directors believes that the Company’s current disclosures provide shareholders with comprehensive information on its political contributions. Pfizer complies fully with all federal, state and local laws, including reporting requirements, governing its corporate political and Political Action Committee (PAC) contributions. Pfizer’s political contributions disclosure policy provides that “[a]ll federal and state contributions and expenditures made by the Company shall be disclosed semi-annually on the Pfizer Inc. website.” This includes contributions to candidates, political committees and political parties, as well as contributions related to ballot measures. The Pfizer PAC and Corporate Political Contributions Report details, by recipient and amount, Pfizer PAC and Pfizer Inc. contributions to political committees, corporate contributions made in state and local elections, and certain contributions to trade associations. The Report also identifies, by name and title, each member of the Political Contributions Policy Committee (PCPC) and Pfizer PAC Steering Committee. The PCPC oversees the day-to-day operations of the PAC, including all PAC solicitations, and the Pfizer PAC Steering Committee reviews and approves all political contribution requests.
| 36 |

|
2012 PROXY STATEMENT |
Table of Contents
SHAREHOLDER PROPOSALS
In addition, Pfizer asks trade associations receiving $100,000 or more from the Company in a given year to report to us the portion of Pfizer dues/payments used for political expenditures/contributions. We voluntarily include this information in the Report and on our website. Prior to publication, the PAC and Corporate Political Contributions Report is presented to the Board. We encourage shareholders to view the report on our corporate website at: www.pfizer.com/about/corporate_governance/political_action_committee_report.jsp.
We regularly re-evaluate our reporting practices to ensure that the Company’s disclosure practices and policies meet the needs of our shareholders and other stakeholders; as part of this process, we speak with representatives from many shareholder and stakeholder groups. In 2011, the Company adopted a policy that prohibits employees from directly making independent expenditures using corporate treasury funds. This type of expenditure, which would permit employees to expressly advocate the election or defeat of a clearly identified candidate, was the subject of the United States Supreme Court’s 2010 decision in Citizens United v. Federal Election Commission. We adopted our policy prohibiting such payments to demonstrate our responsiveness to shareholder concerns prompted by the Supreme Court’s decision.
The Board believes that adopting this proposal is not in the best interests of the Company and its shareholders and, furthermore, that the proponent’s request—specifically, that these contributions be published in certain U.S., local, and national newspapers and additional shareholder reports—would be an unnecessary expenditure of corporate resources and would not be useful to shareholders.
Your Board of Directors unanimously recommends a vote AGAINST this proposal.
ITEM 5 – SHAREHOLDER PROPOSAL REGARDING ACTION BY WRITTEN CONSENT
Mr. William Steiner, 112 Abbottsford Gate, Piermont, New York 10968, who represents that he owns 12,700 shares of Pfizer common stock, has submitted the following proposal for consideration at the Annual Meeting:
RESOLVED, Shareholders request that our board of directors undertake such steps as may be necessary to permit written consent by shareholders entitled to cast the minimum number of votes that would be necessary to authorize the action at a meeting at which all shareholders entitled to vote thereon were present and voting (to the fullest extent permitted by law). This includes written consent regarding issues that our board is not in favor of.
This proposal topic won majority shareholder support at 13 major companies in 2010. This included 67%-support at both Allstate and Sprint. Hundreds of major companies enable shareholder action by written consent.
Taking action by written consent in place of a meeting is a means shareholders can use to raise important matters outside the normal annual meeting cycle. A study by Harvard professor Paul Gompers supports the concept that shareholder dis-empowering governance features, including restrictions on shareholder ability to act by written consent, are significantly related to reduced shareholder value.
In spite of our company trying to create the impression that it is shareholder-friendly, our company used corporate money to tilt the vote against widely-supported shareholder proposals in 2011. This included shareholder proposals for a shareholder right to act by written consent and a shareholder proposal for 10% of shareholders to call a special meeting. As a result the strong 2011 shareholder support for these topics was probably understated.
Please encourage our board to respond positively to this proposal to support improved corporate governance and financial performance: Shareholder Action by Written Consent—Yes on 5.
| 2012 PROXY STATEMENT |

|
37 |
Table of Contents
SHAREHOLDER PROPOSALS
| Your Company’s Response: |
The Board of Directors believes that important matters should be the subject of the annual meeting of shareholders or a special meeting of shareholders, either of which would provide the opportunity for discussion and interaction among the Company’s shareholders so that all points of view may be considered prior to a vote. Special meetings of shareholders, which can be called by either the Board or shareholders representing 20% of the outstanding Pfizer common stock, help ensure that significant corporate actions are taken when there is a clear consensus that such action is prudent and in the best interests of shareholders. This approach also helps ensure that the Company governs its affairs in the fairest, most efficient and cost-effective manner consistent with legal, regulatory and internal requirements.
In contrast, this proposal would allow critical actions to be approved without the benefit of a meeting and without proper notice to all shareholders and the Company. If adopted, we believe this proposal could disenfranchise many shareholders—particularly smaller shareholders—on potentially critical matters that should be presented at an appropriately called annual or special meeting. At the 2010 annual meeting, a Company-sponsored resolution to reduce the percentage of shares required for shareholders to call a special meeting passed overwhelmingly. That vote demonstrates that shareholders consider the ability to call a special meeting an important part of shareholder empowerment, which could be jeopardized by this proposal.
This proposal also should be evaluated in the context of the Company’s overall corporate governance record. Pfizer is a leader in providing opportunities for active engagement with shareholders. More importantly, the Board has not merely listened to shareholders; it has acted on their suggestions and implemented a number of their recommendations. Actions such as eliminating the “poison pill,” super-majority vote requirements and the classified board; embracing majority voting for Directors; and, more recently, allowing shareholders to call special meetings, demonstrate the Company’s ongoing commitment to good governance practices. The Company takes pride in its responsiveness to shareholders and its status as a leader in good governance, and we believe in maintaining policies and practices that serve the interests of all shareholders. This proposal would not do so.
Your Board of Directors unanimously recommends a vote AGAINST this proposal.
ITEM 6 – SHAREHOLDER PROPOSAL REGARDING SPECIAL SHAREHOLDER MEETINGS
Mr. Kenneth Steiner, 14 Stoner Avenue, 2M, Great Neck, New York 11021, who represents that he owns 1,240 shares of Pfizer common stock, has submitted the following proposal for consideration at the Annual Meeting:
RESOLVED, Shareowners ask our board to take the steps necessary unilaterally (to the fullest extent permitted by law) to amend our bylaws and each appropriate governing document to give holders of 10% of our outstanding common stock (or the lowest percentage permitted by law above 10%) the power to call a special shareowner meeting.
This includes that such bylaw and/or charter text will not have any exclusionary or prohibitive language in regard to calling a special meeting that apply only to shareowners but not to management and/or the board (to the fullest extent permitted by law).
Special meetings allow shareowners to vote on important matters, such as electing new directors that can arise between annual meetings. Shareowner input on the timing of shareowner meetings is especially important when events unfold quickly and issues may become moot by the next annual meeting. This proposal does not impact our board’s current power to call a special meeting.
This proposal topic won more than 60% support at CVS, Sprint and Safeway.
| 38 |

|
2012 PROXY STATEMENT |
Table of Contents
SHAREHOLDER PROPOSALS
In spite of our company trying to create the impression that it is shareholder-friendly, our company used corporate money to tilt the vote against widely-supported shareholder proposals in 2011. This included shareholder proposals for a shareholder right to act by written consent and a shareholder proposal for 10% of shareholders to call a special meeting. As a result the strong 2011 shareholder support for these topics was probably understated.
Please encourage our board to respond positively to this proposal to initiate improved corporate governance and financial performance: Special Shareowner Meetings—Yes on 6.
| Your Company’s Response: |
Shareholder meetings are serious events that consume significant corporate time and resources. Preparing for a shareholder meeting requires substantial attention from Pfizer’s Board of Directors, officers and employees, thus diverting attention away from their primary function of operating the business in the best interests of the shareholders. Allowing a small minority of shareholders, including those who could borrow shares from other shareholders in order to vote on a particular issue, to call special meetings for any reason could be detrimental to shareholders. Accordingly, the Board believes it is important that the ownership threshold to call special meetings strike the right balance between enhancing shareholder rights and protecting against the risk that a small minority of shareholders, including those with special interests, could call special meetings, with the resulting expense and disruption to our business.
The Board has been considering this subject, and discussing it with shareholders, since 2008. Based on those discussions, the Board continues to believe that the current 20% ownership threshold to call special meetings strikes a reasonable and appropriate balance of the above factors.
Your Board of Directors unanimously recommends a vote AGAINST this Proposal.
ITEM 7 – SHAREHOLDER PROPOSAL REGARDING ADVISORY VOTE ON DIRECTOR PAY
Ray T. Chevedden, 5965 S. Citrus Ave., Los Angeles, California 90043, who represents that he owns
200 shares of Pfizer common stock, has submitted the following proposal for consideration at the
Annual Meeting:
Resolved: Shareholders request that our Board of Directors adopt a policy that provides shareholders the opportunity, at each annual meeting, to vote on an advisory proposal, prepared by the Board of Directors, to ratify the pay given members of our Board of Directors as disclosed in the proxy statement. The proposal submitted to shareholders should make clear that the vote is non-binding and would not affect any pay given to any director.
The proxy advisory firms Institutional Shareholder Services and Glass Lewis each recommended that shareholders of at least one major company vote in favor of a 2011 shareholder proposal on this topic. A shareholder proposal with similarities to this proposal won 55%-support at a major company in 2010.
This proposal is similar to our management’s proposal on this same ballot enabling us to cast a vote in regard to the pay of our executives. This shareholder proposal simply extends the shareholder voting opportunity to apply to our directors.
The merit of this proposal should also be considered in the context of the opportunity for additional improvement in our company’s 2011 reported corporate governance in order to more fully realize our company’s potential:
The Corporate Library, an independent research firm rated our company “D” with “High Concern” for executive pay—$20 million for former CEO Jeffrey Kindler. The 2010 increase in pension amount for our CEO Ian Read was $10 million. Apparently as a result of these excesses each member of our executive pay committee received our highest negative votes.
| 2012 PROXY STATEMENT |

|
39 |
Table of Contents
SHAREHOLDER PROPOSALS
Three of our directors had long-tenure of 15 to 23 years. These long-tenure directors held 6 seats on our most important board committees. This included Don Cornwell’s chairmanship of our Audit Committee. Long-tenured directors can often form relationships that may compromise their independence and therefore hinder their ability to provide effective oversight.
William Gray was designated as a “Flagged (Problem) Director” by The Corporate Library because of his responsibilities on the Visteon board leading up to its bankruptcy. Mr. Gray was even allowed on our nomination committee.
In spite of our company trying to create the impression that it is shareholder-friendly, our company used corporate money to tilt the vote against widely-supported shareholder proposals in 2011. This included shareholder proposals for a shareholder right to act by written consent and a shareholder proposal for 10% of shareholders to call a special meeting. As a result the strong 2011 shareholder support for these topics was probably understated.
The above concerns shows there is need for improvement. Please encourage our board to respond positively to this proposal: Shareholder Say on Director Pay—Yes on 7.
| Your Company’s Response: |
The Board of Directors believes that Pfizer’s compensation program for non-employee Directors is reasonable and appropriate for a company of Pfizer’s size and scope, and justified in view of the substantial amounts of time Directors devote to the Company throughout the year. The program provides that Directors receive 50% of their compensation for Board service in the form of equity that may not be disposed of until a Director retires from the Board, thus aligning the interests of the Directors with those of our shareholders. Moreover, there is a robust process for evaluating non-employee Director compensation, including review by an independent consulting firm.
Furthermore, the Board believes that its corporate governance practices provide numerous ways for shareholders to express their views to the Directors on the matters noted in the supporting statement or otherwise. For example, all Directors are elected annually; to be elected in an uncontested election, a Director must receive a majority of votes cast; and shareholders and other stakeholders can contact our Directors through a variety of methods. In addition, notwithstanding the claims made in the supporting statement, each of our Directors who stood for election at the 2011 Annual Meeting received more than 93% of the votes cast at the Meeting.
Neither this proposal nor the proponent’s supporting statement provides any rationale supporting the submission of Director compensation to an advisory vote. The proponent notes that in 2010 a similar proposal received 55% of the votes cast by shareholders of a “major company” and that in 2011 certain proxy advisory firms recommended that shareholders of “at least one major company” vote in favor of a proposal on this topic. However, no company is identified, nor does the proponent even suggest that any such company has any characteristics similar to those of Pfizer. In fact, neither the proposal nor the supporting statement provides any indication that our Directors receive inappropriate or excessive compensation.
Finally, there is no connection between the proponent’s criticisms and Director compensation, nor does the proposal or the supporting statement indicate how any of the criticisms would be addressed—much less remedied—by the proposed advisory vote.
Your Board of Directors unanimously recommends a vote AGAINST this proposal.
| 40 |

|
2012 PROXY STATEMENT |
Table of Contents
Table of Contents
| 42 | ||||
| 46 | ||||
| 47 | ||||
| 47 | ||||
| 47 | ||||
| 48 | ||||
| 49 | ||||
| 50 | ||||
| 51 | ||||
| 52 | ||||
| 52 | ||||
| 52 | ||||
| 53 | ||||
| 54 | ||||
| 54 | ||||
| 54 | ||||
| 55 | ||||
| 55 | ||||
| 56 | ||||
| 57 | ||||
| 57 | ||||
| 58 | ||||
| 58 | ||||
| 59 | ||||
| 59 | ||||
| Philosophy, Goals and Principles of Our Executive Compensation Program |
59 | |||
| 59 | ||||
| 59 | ||||
| 59 | ||||
| 60 | ||||
| 61 | ||||
| 61 | ||||
| 61 | ||||
| 61 | ||||
| 61 | ||||
| 62 | ||||
| 64 | ||||
| 64 | ||||
| 65 | ||||
| 67 | ||||
| 67 | ||||
| 67 | ||||
| 67 | ||||
| 67 | ||||
| 68 | ||||
| 69 | ||||
| 70 | ||||
| 70 | ||||
| 71 | ||||
| 72 | ||||
| 74 | ||||
| 75 | ||||
| 76 | ||||
| 78 | ||||
| 79 | ||||
| 80 | ||||
| 81 | ||||
| 2012 PROXY STATEMENT |

|
41 |
Table of Contents
Pfizer’s Pay for Performance Philosophy, Goals and Principles
Pfizer’s compensation philosophy, which is set by the Compensation Committee, is to align each executive’s compensation with Pfizer’s short-term and long-term performance and to provide the compensation and incentives needed to attract, motivate and retain key executives who are crucial to Pfizer’s long-term success.
The Global Performance Plan (“GPP”), our annual incentive program, is funded based on Pfizer’s performance on three financial metrics: total revenue, adjusted diluted earnings per share, and cash flow from operations. The GPP pool is not funded unless performance exceeds a threshold level of performance. Individual awards are earned based on the available earned pool, Business Unit/Function performance, and the achievement of annual performance objectives for the individual.
Our annual long-term incentive awards are aligned with the interests of our shareholders because they deliver value based on absolute and relative shareholder return, encourage stock ownership and promote retention of key talent.
A significant portion of the total compensation opportunity for each of our executives (including the Named Executive Officers, or “NEOs”) is directly related to Pfizer’s stock price performance and to other performance factors that measure our progress against the goals of our strategic and operating plans, as well as our performance against that of our pharmaceutical peer group.
2011 PERFORMANCE OVERVIEW
2011 was a year marked by ongoing change throughout Pfizer brought about by a difficult market environment, increased pricing pressures and the loss of exclusivity of Lipitor. We were driven by our commitment to be a focused, innovative biopharmaceutical company positioned to deliver value for our shareholders. Under the leadership of Ian C. Read, we set a course to redefine and strengthen Pfizer, driven by four imperatives:
Improving the performance of our innovative core:
| • | We reduced our adjusted Research and Development spend for 2011 by nearly a billion dollars compared to 2010 and increased the focus of our R&D investments to our high-priority therapeutic areas including Cardiovascular, Metabolic and Neuroendocrine Diseases, Immunology and Inflammation, Neuroscience and Pain, Oncology and Vaccines. |
| • | We saw steady progress in our late stage pipeline with over five approvals in the U.S. and E.U. |
| • | We advanced approximately 30 programs in our early- and mid-stage (Phase I and II) pipeline. |
| • | We continued to invest in our R&D network and the capabilities designed to drive biomedical innovation. |
Maximizing capital allocation and growth opportunities:
| • | We met or exceeded all elements of 2011 financial guidance. |
| • | We generated $1.5 billion of operating cash flow incremental to the 2011 operating plan through various finance and business operations initiatives. |
| • | We returned $15.2 billion in capital to shareholders through dividend payments and share repurchases. |
| • | We completed the divestiture of Capsugel and successfully closed the acquisition of King Pharmaceuticals, Inc. and Ferrosan’s Consumer Health business. |
| 42 |

|
2012 PROXY STATEMENT |
Table of Contents
EXECUTIVE SUMMARY
Earning respect from society:
| • | We continued to help qualified uninsured and underinsured patients access medicines for free or at a savings through the “Pfizer Helpful Answers” program in the U.S. |
| • | Globally, we extended our commitment to help nations achieve the UN Millennium Goals, particularly in Healthcare. |
| • | We engaged government leaders on the value of our medicines and our contribution to the economy. |
Creating an ownership culture:
| • | We designed a culture model to encourage employee ownership, collaboration and initiative; to build a strong, engaged leadership team; and to develop key talent. |
| • | We continued to develop diverse talent at senior levels and in the talent pipeline. |
ELEMENTS OF EXECUTIVE COMPENSATION
| ELEMENT | TYPE | TERMS | ||
| Annual Long-Term Incentive Compensation (100% Equity) |
Restricted Stock Units (RSUs)
(representing 25% of total annual grant value) |
•RSUs generally vest three years from the grant date
•Dividend equivalent units (DEUs) are accumulated on RSUs during the vesting period
•Both RSUs and DEUs are paid in shares of Pfizer common stock and only on vesting* | ||
| 5- and 7-Year Total Shareholder Return Units (5-Year and 7-Year TSRUs)
(each representing 25% of total annual grant value) |
•5- and 7-Year TSRUs generally vest three years from the grant date and are settled five or seven years from the grant date, respectively
•Dividend equivalents are accumulated on TSRUs during the five- or seven-year term
•The number of shares that may be earned for each TSRU is equal to the difference between the settlement price (the 20-day average of the closing prices of Pfizer common stock prior to settlement) and the grant price (the closing price of Pfizer common stock on the date of grant) plus the value of dividend equivalents accumulated over the term, subject to the results being positive
•Both 5- and 7-Year TSRUs are paid in shares of Pfizer common stock on settlement | |||
| Performance Share Awards (PSAs)
(representing 25% of total annual grant value) |
•PSAs generally vest three years from the grant date
•The performance period for PSAs is three years
•The number of shares that may be earned over the performance period is based on Pfizer’s Total Shareholder Return (TSR, defined as change in stock price plus dividends) relative to the TSR of our pharmaceutical peer group (see “Performance Share Awards”) and ranges from 0% to 200% of the initial award
•Dividend equivalents are applied to the number of shares actually earned under the award
•PSAs are paid in shares of Pfizer common stock | |||
|
Cash |
Salary | •The fixed amount of compensation for performing day-to-day responsibilities. Generally eligible for increase annually, depending on market movement, performance and internal equity | ||
| Annual Short-Term Incentive/GPP | •Provides the opportunity for competitively-based annual incentive awards for achieving Pfizer’s short-term financial goals and other strategic objectives measured over the current year | |||
|
Retirement |
Pension | •Provides retirement income for eligible participants based on years of service and highest average earnings up to tax code limitations | ||
| Supplemental Pension | •Provides retirement income, on a non-qualified basis, relating to compensation in excess of tax code limitations under the same formula as the qualified pension noted above | |||
| Savings Plan | •A qualified 401(k) plan that provides participants with the opportunity to defer a portion of their compensation, up to tax code limitations, and receive a company matching contribution | |||
| Supplemental Savings Plan | •Extends the Savings Plan, on a non-qualified basis, for compensation in excess of the tax code limitations under the same terms | |||
| Other |
Perquisites | •Certain other benefits provided to executives by the Company |
| * | Unless automatically deferred due to Section 162(m) of the Internal Revenue Code (the “IRC”). |
| 2012 PROXY STATEMENT |

|
43 |
Table of Contents
EXECUTIVE SUMMARY
| 44 |

|
2012 PROXY STATEMENT |
Table of Contents
EXECUTIVE SUMMARY
RECENT COMMITTEE ACTIONS
We took a number of actions during 2011 to make our executive compensation program more reflective of our performance and more responsive to shareholder interests. These actions included the following:
| TOPIC | ACTION |
RATIONALE | ||
| Frequency of future advisory votes on executive compensation | Adopted annual advisory vote on executive compensation | To be responsive to shareholder preference for annual advisory votes | ||
| Annual Short-Term Incentive (GPP) pool |
After review, confirmed that the metrics used to fund the pool continue to support the Company’s annual operating plan; revised weightings from 33-1/3% each to 40% for total revenue, 40% for adjusted diluted EPS and 20% for cash flow from operations | Revenue is a leading indicator of performance and value creation; EPS is a strong indicator of sustained performance over the long term; cash flow generates cash to fund short-term operations and to fund dividends and stock repurchases, and encourages expense control | ||
| Annual Executive Long-Term Incentive Program | Beginning in 2011, denominated 25% of long-term incentive value as 7-Year Total Shareholder Return Units (TSRUs), replacing Short-Term Incentive Shift Awards (STI Shift Awards) | Consistent with the intent to grant STI Shift Awards only during a limited period (2008-2010), 7-Year TSRUs more closely align with long-term shareholder interests since these Units provide value based on Pfizer’s total shareholder return over a seven-year performance period | ||
| Worldwide Research and Development (WRD) Portfolio Performance Share Long-Term Incentives | For grants commencing in 2012, designed a new long-term incentive vehicle for eligible WRD colleagues in the form of Portfolio Performance Shares, with payouts based on the achievement of WRD performance goals supporting the pipeline portfolio; members of the Executive Leadership Team, including the NEOs, do not participate in the WRD program | Closer alignment with WRD’s strategy to drive sustained progress on the product portfolio and create shareholder value | ||
| Share Ownership Requirements | Effective in 2011, increased share ownership requirements for the CEO to 6 times base salary from 5 times base salary |
Consistent with leading practices and shareholder advisory group standards | ||
| Compensation Committee Charter |
Revised the Charter to include responsibility for consulting with and considering the recommendations of the Regulatory and Compliance Committee regarding “clawbacks” of incentive compensation | Responsive to shareholder concerns; supports ongoing compliance responsibilities and aligned with Regulatory and Compliance Committee Charter | ||
| Performance Share Awards (PSAs) | Effective with grants commencing in 2012, revised the method for calculating Total Shareholder Return for purposes of evaluating performance under PSAs from single end-to-end closing stock prices to the 20-day average closing stock prices prior to the beginning and end of the performance periods and adjusted the payout matrix to better align with performance | Aligned with performance and market practice; minimizes the effect of a single day stock price volatility | ||
| 2012 PROXY STATEMENT |

|
45 |
Table of Contents
The Compensation Committee has reviewed and discussed with management the following Compensation Discussion and Analysis section of the Company’s 2012 Proxy Statement. Based on our review and discussions, we have recommended to the Board of Directors that the Compensation Discussion and Analysis be included in Pfizer’s 2012 Proxy Statement.
The Compensation Committee
|
|
|||
| James M. Kilts, Chair | Frances D. Fergusson | |||
|

|
|||
| W. Don Cornwell | Suzanne Nora Johnson | |||
| 46 |

|
2012 PROXY STATEMENT |
Table of Contents
| 2012 PROXY STATEMENT |

|
47 |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
We took a number of actions during 2011 to make our executive compensation program more reflective of our performance and more responsive to shareholder interests. These actions included the following:
| TOPIC | ACTION | RATIONALE | ||
| Frequency of future advisory votes on executive compensation | Adopted annual advisory vote on executive compensation | To be responsive to shareholder preference for annual advisory votes | ||
| Annual Short-Term Incentive (GPP) pool | After review, confirmed that the metrics used to fund the pool continue to support the Company’s annual operating plan; revised weightings from 33-1/3% each to 40% for total revenue, 40% for adjusted diluted EPS and 20% for cash flow from operations | Revenue is a leading indicator of performance and value creation; EPS is a strong indicator of sustained performance over the long term; cash flow generates cash to fund short-term operations and to fund dividends and stock repurchases, and encourages expense control | ||
| Annual Executive Long-Term Incentive Program | Beginning in 2011, denominated 25% of long-term incentive value as 7-Year Total Shareholder Return Units (TSRUs), replacing Short-Term Incentive Shift Awards (STI Shift Awards) | Consistent with the intent to grant STI Shift Awards only during a limited period (2008-2010), 7-Year TSRUs more closely align with long-term shareholder interests since these units provide value based on Pfizer’s total shareholder return over a seven-year performance period | ||
| Worldwide Research and Development (WRD) Portfolio Performance Share Long-Term Incentives | For grants commencing in 2012, designed a new long-term incentive vehicle for eligible WRD colleagues in the form of Portfolio Performance Shares, with payouts based on the achievement of WRD performance goals supporting the pipeline portfolio; members of the Executive Leadership Team, including the NEOs, do not participate in the WRD program | Closer alignment with WRD’s strategy to drive sustained progress on the product portfolio and create shareholder value | ||
| Share Ownership Requirements | Effective in 2011, increased share ownership requirements for the CEO to 6 times base salary from 5 times base salary | Consistent with leading practices and shareholder advisory group standards | ||
| Compensation Committee Charter |
Revised the Charter to include responsibility for consulting with and considering the recommendations of the Regulatory and Compliance Committee regarding “clawbacks” of incentive compensation | Responsive to shareholder concerns; supports ongoing compliance responsibilities and aligned with Regulatory and Compliance Committee Charter | ||
| Performance Share Awards (PSAs) |
Effective with grants commencing in 2012, revised the method for calculating Total Shareholder Return for purposes of evaluating performance under PSAs from single end-to-end closing stock prices to the 20-day average closing stock prices prior to the beginning and end of the performance periods and adjusted the payout matrix to better align with performance | Aligned with performance and market practice; minimizes the effect of a single day stock price volatility | ||
| 48 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
| 2012 PROXY STATEMENT |

|
49 |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
Pfizer continues to implement and maintain leading practices in its compensation program and related areas. These practices include the following:
| • | We prohibit our executives and Directors from hedging, or engaging in any derivatives trading, with respect to Company shares (see “Derivatives Trading” below). |
| • | We do not provide tax “gross-ups” for perquisites provided to our executive officers, other than in the case of certain relocation expenses, consistent with our relocation policy (see “Perquisites” below). |
| • | We require our executive officers to meet stock ownership requirements, and we prohibit them from selling any shares (except to meet tax withholding obligations) if doing so would cause them to fall below required levels (see “Stock Ownership and Holding Requirements” below). Effective January 1, 2011, the ownership requirement for our CEO was increased to 6 times base salary from 5 times base salary. We also have stock ownership requirements for our Directors, as discussed elsewhere in this Proxy Statement. |
| • | Our equity incentive plan prohibits the repricing or exchange of equity awards without shareholder approval. |
| • | Our annual equity awards provide for minimum three-year vesting, except in limited circumstances involving certain terminations of employment, and we have not granted stock options to executive officers since 2007. |
| • | None of our executive officers has an employment agreement with the Company. |
| • | To the extent permitted by law, we can recover cash- or equity-based compensation paid to executives in various circumstances, including where the compensation is based upon the achievement of specified financial results that are the subject of a subsequent restatement (see “Compensation Recovery” below). |
| • | Our executive compensation program includes a number of controls that mitigate risk, including executive stock ownership requirements and, under certain circumstances, our ability to recover compensation paid to executives, each as mentioned above. |
| • | The Committee has engaged an independent compensation consultant that has no other ties to the Company or its management and that meets stringent selection criteria (see “Role of Compensation Consultant” below). |
| • | We maintain a robust investor outreach program that enables us to obtain ongoing feedback concerning our compensation program, as well as how we disclose that program. In 2011, as has been the case for many years, we not only listened to our investors’ views; we actively sought out those views and welcomed and implemented a number of their suggestions (see “Response to 2011 Say on Pay Vote and Shareholder Outreach” above). |
| 50 |

|
2012 PROXY STATEMENT |
Table of Contents
ELEMENTS OF EXECUTIVE COMPENSATION
| ELEMENT | TYPE | TERMS | ||
| Annual Long-Term Incentive Compensation (100% Equity) |
Restricted Stock Units
(representing 25% of total annual grant value) |
•RSUs generally vest three years from the grant date
•Dividend equivalent units (DEUs) are accumulated on RSUs during the vesting period
•Both RSUs and DEUs are paid in shares of Pfizer common stock and only on vesting* | ||
| 5- and 7-Year Total Shareholder Return Units
(each representing 25% of total annual grant value) |
•5- and 7-Year TSRUs generally vest three years from the grant date and are settled five or seven years from the grant date, respectively
•Dividend equivalents are accumulated on TSRUs during the five- or seven-year term
•The number of shares that may be earned for each TSRU is equal to the difference between the settlement price (the 20-day average of the closing prices of Pfizer common stock prior to settlement) and the grant price (the closing price of Pfizer common stock on the date of grant) plus the value of dividend equivalents accumulated over the term, subject to the results being positive
•Both 5- and 7-Year TSRUs are paid in shares of Pfizer common stock on settlement | |||
| Performance Share Awards
(representing 25% of total annual grant value) |
•PSAs generally vest three years from the grant date
•The performance period for PSAs is three years
•The number of shares that may be earned over the performance period is based on Pfizer’s Total Shareholder Return (TSR, defined as change in stock price plus dividends) relative to the TSR of our pharmaceutical peer group (see “Performance Share Awards”) and ranges from 0% to 200% of the initial award
•Dividend equivalents are applied to the number of shares actually earned under the award
•PSAs are paid in shares of Pfizer common stock | |||
|
Cash |
Salary | •The fixed amount of compensation for performing day-to-day responsibilities. Generally eligible for increase annually, depending on market movement, performance and internal equity | ||
| Annual Short-Term Incentive/GPP | •Provides the opportunity for competitively-based annual incentive awards for achieving Pfizer’s short-term financial goals and other strategic objectives measured over the current year | |||
|
Retirement |
Pension | •Provides retirement income for eligible participants based on years of service and highest average earnings up to tax code limitations | ||
| Supplemental Pension | •Provides retirement income, on a non-qualified basis, relating to compensation in excess of tax code limitations under the same formula as the qualified pension noted above | |||
| Savings Plan | •A qualified 401(k) plan that provides participants with the opportunity to defer a portion of their compensation, up to tax code limitations, and receive a company matching contribution | |||
| Supplemental Savings Plan | •Extends the Savings Plan, on a non-qualified basis, for compensation in excess of the tax code limitations under the same terms | |||
| Other |
Perquisites | •Certain other benefits provided to executives by the Company |
| * | Unless automatically deferred due to IRC Section 162(m). |
| 2012 PROXY STATEMENT |

|
51 |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
KEY COMPENSATION ACTIONS FOR 2011
The following highlights the Committee’s key compensation decisions for 2011, as reported in the 2011 Summary Compensation Table. These decisions were made with the advice of the Committee’s independent consultant, Frederic W. Cook & Co. (see “Role of Compensation Consultant” below), and are discussed in greater detail elsewhere in this CD&A.
Effective January 1, 2011, in connection with the election of Mr. Read as President and CEO in December 2010, the Committee adjusted his salary grade to reflect the change in his position to CEO and made a number of adjustments to his compensation effective January 1, 2011.
| • | His base salary was set at $1.7 million; |
| • | His annual incentive target award increased to $2.6 million; and |
| • | His annual long-term incentive target award value increased to $10.0 million. |
These adjustments were reviewed in detail by the Committee and its independent consultant. Approximately 88% of Mr. Read’s compensation opportunity was tied to Company and individual performance, based upon target annual incentive and long-term award values. The factors considered by the Committee in determining Mr. Read’s compensation are discussed under “Evaluating Performance”.
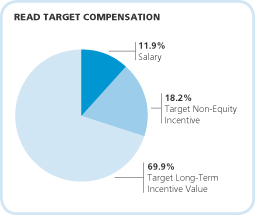
In 2011, 90% of Mr. Read’s actual total direct compensation was performance-based.
Compensation for Our Other Named Executive Officers
The Committee also made compensation adjustments for the other NEOs in January 2011. These adjustments were based upon the recommendations of the CEO, evaluation by the Committee and the other independent members of the Board of each individual’s performance (see “Evaluating Performance”), the advice of the Committee’s independent consultant, salary data from the peer and comparator groups, internal pay relationships based on relative duties and responsibilities, the individual’s future advancement potential, his or her impact on Pfizer’s results and for retention purposes. Based upon these considerations, the Committee adjusted the salary grades and made a number of adjustments to the target compensation of our other NEOs listed below, in all cases reflecting their assumption of additional responsibilities, as well as the factors listed above.
| 52 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
Approximately 80% of the compensation opportunity for our other NEOs was tied to Company and individual performance, based upon target annual incentive and long-term award values. The factors considered by the Committee in determining compensation for our other NEOs are discussed below (see “Evaluating Performance”).
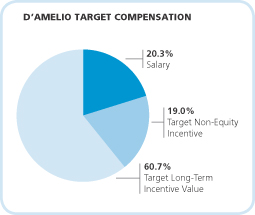
|
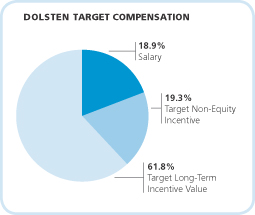
|
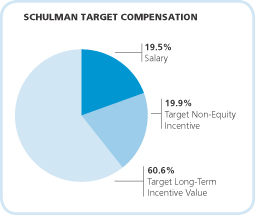
|
|
The table below shows the annual salaries set by the Committee, effective January 1, 2011.
| NAME | SALARY EFFECTIVE 1/1/11 | 2011 SALARY GRADE MIDPOINT(1) | ||||||
| I. Read |
$ | 1,700,000 | $ | 1,725,000 | ||||
| F. D’Amelio |
$ | 1,200,000 | $ | 1,125,000 | ||||
| M. Dolsten |
$ | 1,100,000 | $ | 1,125,000 | ||||
| A. W. Schulman |
$ | 900,000 | $ | 1,020,000 | ||||
| D. Simmons |
$ | 850,000 | $ | 1,020,000 | ||||
| (1) | See “Annual Incentive Awards (Cash)” for an explanation of how we use salary grade midpoints to determine target annual incentive awards. |
| 2012 PROXY STATEMENT |

|
53 |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
Annual Incentive Compensation Criteria
Annual incentives for each ELT member, including our NEOs, are based on:
| • | The financial performance of the Company measured by total revenue, adjusted diluted EPS and cash flow from operations; |
| • | The financial performance of the executive’s Business Unit/Function measured by: revenue and income before adjustments; |
| • | The achievement of selected strategic and operational goals for the executive’s Business Unit/Function; and |
| • | The Committee’s assessment of the executive’s individual performance. |
Each year, the Committee evaluates the continued use of the financial measures that fund the annual incentive pool, using these basic principles:
| • | measures that support achieving the Company’s annual operating plan; |
| • | measures that promote decisions and behaviors aligned with maximizing near-term business results while supporting the achievement of the Company’s long-term goals; |
| • | measures that exhibit a strong line of sight (i.e., are clearly understood and can be impacted by the performance of our executives and employees); and |
| • | measures that are consistent with best practices and are commonly used within our industry. |
|
The Committee believes that the continued use of these financial measures supports these basic principles:
• Revenue is a leading indicator of performance and value creation; provides a clear focus on growth; is an important measure in a sales industry; and is understandable with clear line of sight and employee impact.
• EPS is a comprehensive measure of income; provides focus on profitable growth; focuses managers on expense control; is viewed as a strong indicator of sustained performance over the long term; is understandable with clear line of sight and employee impact.
• Cash flow provides focus on generating cash in the short term to fund operations and research and to return funds to shareholders in the form of dividends and share repurchases; focuses managers on expense control; and is a strong link to long-term shareholder value creation.
|
As in prior years, the Committee considered other metrics such as Return on Equity, Return on Assets, Return on Invested Capital and economic value added as potential measures under our short-term plan, but determined that the metrics selected were better suited for a biopharmaceutical company with long lead times and uncertainties relating to product development. The Committee also believes that the alternative metrics lacked clear line of sight for employees and are not appropriate measures for Pfizer’s short-term plan.
The target annual incentive award opportunity for our NEOs represents a percentage of salary grade midpoint. Target annual incentive award levels are reviewed annually to ensure alignment with our compensation philosophy to target each compensation element and total direct compensation at the market median and are based on an evaluation of competitive market data and internal equity among the members of our ELT, the executives who report directly to the CEO. For 2011, target annual incentive opportunities for the NEOs ranged from 90%–150% of salary midpoint, as indicated in the table below.
Financial Results for Annual Incentive Purposes
The annual incentive awards were based on both individual performance and the Company’s strong 2011 operating performance, which exceeded the target goals for 2011 set by the Committee for annual incentive purposes. These targets for compensation purposes were set by the Committee based on its evaluation of the budget amounts and its determination that there was a sufficient degree of stretch in the targets.
| FINANCIAL OBJECTIVES | 2010 RESULTS | 2011 THRESHOLD | 2011 TARGET | 2011 RESULTS | ||||||||||||
| Total Revenue(a) |
$ | 67.4 Billion | $ | 62.2 Billion | $ | 66.8 Billion | $ | 67.0 Billion | ||||||||
| Adjusted Diluted EPS(b) |
$ | 2.26 | $ | 1.99 | $ | 2.20 | $ | 2.27 | ||||||||
| Cash Flow from Operations(c) |
$ | 7.1 Billion | $ | 13.3 Billion | $ | 16.8 Billion | $ | 17.5 Billion | ||||||||
| (a) | Total Revenue for annual incentive purposes is based on budgeted foreign exchange rates. Therefore, 2011 and 2010 results differ from U.S. GAAP revenue of $67.4 billion and $67.1 billion, respectively. See “Financial Measures” for a reconciliation of U.S. GAAP revenue to Total Revenue for 2011 and 2010 for annual incentive purposes. |
| (b) | Adjusted Diluted EPS for annual incentive purposes is based on budgeted foreign exchange rates and excludes certain non-recurring items. See “Financial Measures” for a reconciliation of U.S. GAAP Diluted EPS to the Adjusted Diluted EPS for 2011 and 2010 for annual incentive purposes. |
| (c) | 2011 Targets and Results exclude certain tax and other discretionary timing items for compensation purposes (non-GAAP amounts). |
| 54 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
See “Financial Measures” for reconciliations of 2011 and 2010 U.S. GAAP revenues and U.S. GAAP diluted EPS to Total Revenue and Adjusted Diluted EPS for annual incentive purposes. Adjusted Diluted EPS is defined as U.S. GAAP diluted EPS excluding purchase-accounting adjustments, acquisition-related costs, discontinued operations and certain significant items. Total Revenue and Adjusted Diluted EPS for annual incentive purposes are not, and should not be viewed as, substitutes for U.S. GAAP revenues and U.S. GAAP diluted EPS, respectively.
For annual incentive awards to be deductible under IRC Section 162(m), the total amount of any annual incentive that can be paid to an executive officer in any one year is limited to a maximum of 0.3% of Pfizer’s “adjusted net income” (defined for this purpose as operating income from continuing operations, reduced by taxes and interest expense, and adjusted for any one-time gains or other non-recurring events). Since actual incentive amounts are based on Pfizer’s performance and the Committee’s assessment of each executive’s level of achievement against his or her specified goals, an executive’s annual incentive award may be more or less than target, subject to the overall adjusted net income limitation (see “Evaluating Performance” for a more complete description of how Company and individual performance is evaluated against stated objectives and “Other Compensation Policies—Tax Policies” for further information on our policy on IRC Section 162(m)).
Annual Incentive Awards (Cash)
Annual incentives for 2011 were determined in February 2012. The 2011 awards for the NEOs were paid at an average of 130% of target as compared to 125% for all NEOs in 2010. The Committee reviewed Mr. Read’s performance for 2011 (see “Evaluating Performance”), with input from the other independent members of the Board and with advice from the Committee’s independent consultant, and determined his 2011 annual incentive award. Mr. Read submitted 2011 annual incentive award recommendations to the Committee for each of the other ELT members (including the other NEOs), based on his evaluation of their individual performance (see “Evaluating Performance”), and the performance of their respective Business Units/Functions. The Committee, with input from the other independent members of the Board and the Committee’s independent consultant, reviewed these recommendations and considered its evaluation of each executive’s performance, and his/her relative contribution to the Company’s overall performance, to determine the amounts awarded. The recommendations for the CEO and other ELT members (including the NEOs) were ratified by the independent members of the Board.
2011 annual incentive award targets and payout ranges, as well as the actual annual incentive award payouts for each of the NEOs, are shown in the table below. Actual annual incentive awards are determined based on objective performance measures for the Company (see “Financial Results for Annual Incentive Purposes”) and adjusted for individual and Business Unit/Function performance.
2011 Annual Cash Incentive Awards
| NAME | TARGET PAYOUT AS A % OF SALARY MIDPOINT |
PAYOUT RANGE AS A % OF SALARY MIDPOINT |
TARGET AWARD ($) |
MAXIMUM AWARD ($)(1) |
ACTUAL AWARD ($) |
|||||||||||||||
| I. Read |
150% | 0-300% | 2,587,500 | 5,175,000 | 3,500,000 | |||||||||||||||
| F. D’Amelio |
100% | 0-200% | 1,125,000 | 2,250,000 | 1,440,000 | |||||||||||||||
| M. Dolsten |
100% | 0-200% | 1,125,000 | 2,250,000 | 1,490,000 | |||||||||||||||
| A. W. Schulman |
90% | 0-180% | 918,000 | 1,836,000 | 1,190,000 | |||||||||||||||
| D. Simmons |
90% | 0-180% | 918,000 | 1,836,000 | 1,135,000 | |||||||||||||||
| (1) | Maximum award is 200% of target award. |
Long-Term Incentive Awards (Equity)
Long-term compensation for our ELT (including the NEOs) is delivered entirely in the form of equity awards. In February 2011, executives received long-term equity incentive awards consisting of TSRUs, PSAs, and RSUs. Except for Mr. Read, who received an additional grant of 7-Year premium-priced TSRUs in connection with his appointment as President and CEO, the long-term incentive grant value was equally divided among 5- and 7-Year TSRUs, PSAs, and RSUs (see “Elements of Executive Compensation”).
| 2012 PROXY STATEMENT |

|
55 |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
The 2011 grant value of each NEO’s long-term equity incentive award was based on competitive market data, relative duties and responsibilities, the individual’s future advancement potential, his or her impact on Pfizer’s results and for retention purposes and was as follows:
| NAME | 2011 LONG-TERM INCENTIVE AWARD (MILLIONS) | |||||||||||||||||||
| 7-YEAR TSRUs |
5-YEAR TSRUs |
PSAs |
RSUs |
TOTAL AWARD VALUE |
||||||||||||||||
| I. Read |
$ | 4.3 | $ | 2.5 | $ | 2.5 | $ | 2.5 | $ | 11.8 | * | |||||||||
| F. D’Amelio |
$ | 0.9 | $ | 0.9 | $ | 0.9 | $ | 0.9 | $ | 3.6 | ||||||||||
| M. Dolsten |
$ | 0.9 | $ | 0.9 | $ | 0.9 | $ | 0.9 | $ | 3.6 | ||||||||||
| A. W. Schulman |
$ | 0.75 | $ | 0.75 | $ | 0.75 | $ | 0.75 | $ | 3.0 | ||||||||||
| D. Simmons |
$ | 0.7 | $ | 0.7 | $ | 0.7 | $ | 0.7 | $ | 2.8 | ||||||||||
| * | Includes 420,000 premium-priced TSRUs with an estimated value of $1.8 million (see “2011 Grants of Plan-Based Awards Table”). |
Our long-term equity awards are structured to align our executives’ interests with shareholders and to emphasize the Committee’s expectation that our executive officers focus their efforts on improving Pfizer’s total shareholder return, both on an absolute basis (since the value realized from the TSRUs is consistent with the total shareholder return of Pfizer’s shareholders) and on a relative basis (through PSAs, which are earned based on Pfizer’s total shareholder return compared to peer companies in the pharmaceutical industry). RSUs are used for their potential retention value.
Performance Share Awards (PSAs)
The number of shares that may be earned under the PSAs granted in February 2011 is based on a formula comparing Pfizer’s total shareholder return, including reinvestment of dividend equivalents, over a three-year period to our pharmaceutical peer group, which consists of Abbott Laboratories, Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Roche and Sanofi-Aventis. The award is expressed as a percentage of target award as shown in the chart below. At the end of the performance period, the Committee in its sole discretion may adjust the payout range downward to a percentage not less than the bottom of the payout range. In no event will the payout exceed the maximum payout for the respective range.
2011 Performance Share Award Payout Matrix
| TIER | RANKING | PAYOUT RANGE | ||||
|
1 |
1st or 2nd | 166% – 200% | ||||
|
2 |
3rd or 4th | 133% – 166% | ||||
|
3 |
5th or 6th | 100% – 133% | ||||
|
4 |
7th or 8th | 66% – 100% | ||||
|
5 |
9th or 10th | 33% – 66% | ||||
|
6 |
11th or 12th | 0% – 33% | ||||
Note that in response to comments we received in our shareholder outreach activities, the Performance Share Award Payout Matrix for awards granted commencing in 2012 has been revised to provide for a “0%” payout for Tier 6 performance.
The Committee continues to believe that total shareholder return, defined as change in stock price plus dividends, is the most appropriate measure of relative performance in relation to Pfizer’s business objectives and therefore selected relative total shareholder return as the sole performance measure for the 2011-2013 PSA performance cycle. In the Committee’s view, our relative total shareholder return compared with the pharmaceutical peer group remains a strategic priority.
2009 Performance Share Awards
Our 2009 long-term equity incentive grants to our executive officers also included PSAs. The original peer group companies for the 2009 award consisted of: Abbott Laboratories, Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Schering-Plough and Wyeth. However, with the acquisitions of Wyeth and Schering-Plough in 2009, their results would not be representative of the full performance period (2009-2011). Consequently, the Committee eliminated Wyeth and Schering-Plough from the peer group for purposes of measuring Pfizer’s relative performance for the 2009 award, using the remaining eight pharmaceutical peer companies against which payout of the 2009 performance share awards would be determined. The specific performance levels were revised by the Committee to reflect the smaller group at the points shown in the table below to ensure that the value realized under the PSAs would directly correlate to targeted performance for above median performance, lower awards for threshold performance and substantially greater awards for maximum performance. The matrix for the 2009 performance share awards is shown below:
| 56 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
2009 Performance Share Award Relative Performance/Payout Matrix
| PFIZER RELATIVE PERFORMANCE |
MAXIMUM PAYOUT AS A % OF TARGET |
|||
| 1 (highest) |
200% | |||
| 2 |
175% | |||
| 3 |
150% | |||
| 4 |
100% | |||
| 5 |
75% | |||
| 6 |
50% | |||
| 7 (threshold) |
25% | |||
| 8 |
0% | |||
| 9 (lowest) |
0% | |||
Pfizer’s performance over the three-year period (2009-2011) resulted in a ranking of 4th out of 9, with a maximum payout of 100% of target. The Committee approved payouts at 100% of target as shown below:
Performance Share Award Payout for the 2009-2011 Performance Award Cycle
| NAME(1) | TARGET AWARD AT (#) |
TARGET AWARD VALUE AT GRANT(2) ($) |
ACTUAL AWARD SHARES(3) (#) |
ACTUAL AWARD VALUE AT $21.18 PER SHARE(4) ($) |
||||||||||||
| I. Read |
67,822 | 861,339 | 75,251 | 1,593,816 | ||||||||||||
| F. D’Amelio |
67,822 | 861,339 | 75,251 | 1,593,816 | ||||||||||||
|
A. W. Schulman |
33,911 | 430,670 | 37,626 | 796,919 | ||||||||||||
| D. Simmons |
14,977 | 190,208 | 16,618 | 351,969 | ||||||||||||
| (1) | Based on Dr. Dolsten’s hire date, he does not have any 2009 awards vesting under this Program. |
| (2) | This column represents the target award value based on the February 26, 2009 stock price of $12.70. |
| (3) | These amounts include accumulated dividends on 100% of the target award for the three-year period, converted into shares at $21.18 per share. |
| (4) | This column represents the actual award value based on a stock price of $21.18 on February 24, 2012, which vested on February 26, 2012. |
EARLY 2012 COMPENSATION ACTIONS
Salary and Annual Incentive Targets
In February 2012, the Committee approved 2012 salaries and target annual incentive award levels for the NEOs as follows:
2012 Salary and Annual Incentive Targets
| NAME | APRIL 1, 2012 SALARY ($) |
2012 SALARY MIDPOINT ($)(1) |
2012 TARGET ANNUAL INCENTIVE (%) |
2012 TARGET ANNUAL INCENTIVE(2) ($) |
||||||||||||
| I. Read |
1,750,000 | 1,759,500 | 150% | 2,639,300 | ||||||||||||
| F. D’Amelio |
1,225,000 | 1,147,500 | 100% | 1,147,500 | ||||||||||||
| M. Dolsten |
1,130,000 | 1,147,500 | 100% | 1,147,500 | ||||||||||||
|
A. W. Schulman |
925,000 | 1,040,400 | 90% | 936,400 | ||||||||||||
| D. Simmons |
875,000 | 1,040,400 | 90% | 936,400 | ||||||||||||
| (1) | The 2012 salary midpoints were increased approximately 2% to align with the market. |
| (2) | 2012 target annual incentive amounts are based on a percentage of 2012 salary range midpoints. |
| 2012 PROXY STATEMENT |

|
57 |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
2012 Long-Term Equity Incentive Awards
In February 2012, the Committee granted long-term equity incentive awards to the NEOs in consideration of their 2011 performance and their expected future performance. These awards included 5-Year and 7-Year TSRUs, PSAs and RSUs.
2012 Long-Term Equity Incentive Awards
| NAME | PERFORMANCE PERIOD (OR OTHER PERIOD MATURATION OR PAYMENT PERIOD) |
ESTIMATED FUTURE PAYOUTS UNDER
THE SHARE PROGRAM(1) |
5-YEAR GRANT(4) (#) |
7-YEAR GRANT(5) (#) |
RSU GRANT(6) (#) |
|||||||||||||||||||||
| PSA GRANTS | ||||||||||||||||||||||||||
| THRESHOLD(2) (#) |
TARGET(3) (#) |
MAXIMUM(2) (#) |
||||||||||||||||||||||||
| I. Read |
1/1/12 – 12/31/14 | – | 153,157 | 306,314 | 788,835 | 668,724 | 153,157 | |||||||||||||||||||
|
F. D’Amelio |
1/1/12 – 12/31/14 | – | 42,413 | 84,826 | 218,447 | 185,185 | 42,413 | |||||||||||||||||||
|
M. Dolsten |
1/1/12 – 12/31/14 | – | 42,413 | 84,826 | 218,447 | 185,185 | 42,413 | |||||||||||||||||||
|
A. W. Schulman |
1/1/12 – 12/31/14 | – | 32,988 | 65,976 | 169,903 | 144,033 | 32,988 | |||||||||||||||||||
| D. Simmons |
1/1/12 – 12/31/14 | – | 32,988 | 65,976 | 169,903 | 144,033 | 32,988 | |||||||||||||||||||
| (1) | The actual number of shares, if any, that will be paid out at the end of the performance period cannot be determined because the shares earned by the NEOs will be based upon our future performance compared to the future performance of the pharmaceutical peer group. Dividend equivalents on any shares earned will be paid in shares of common stock at the end of the performance period. |
| (2) | To the extent the Company’s performance equals or exceeds the performance of our pharmaceutical peers, varying amounts of shares of common stock, up to the maximum, will be earned. The Committee will apply the matrix (see “Performance Share Awards” elsewhere in this CD&A), subject to negative discretion, to determine the payout, although in no event shall the payout exceed the maximum payout of the respective range. Commencing with awards granted in 2012, the payout for Tier 6 performance is 0%. |
| (3) | The target amounts vary based on the individual’s salary grade at the time of grant. |
| (4) | 5-Year TSRUs vest on the third anniversary of the grant date (February 23, 2015) and will be settled in shares on the fifth anniversary of the grant date (February 23, 2017). The number of shares delivered at settlement, if any, for each TSRU will equal the difference between the settlement price (the average of the closing prices of Pfizer common stock for the 20 trading days ending February 23, 2017) and the TSRU grant price ($21.03), plus dividend equivalents accrued during the life of the TSRU, divided by the settlement price, subject to the results being positive. |
| (5) | 7-Year TSRUs vest on the third anniversary of the grant date (February 23, 2015) and will be settled in shares on the seventh anniversary of the grant date (February 23, 2019). The number of shares delivered at settlement, if any, for each TSRU will equal the difference between the settlement price (the average of the closing prices of Pfizer common stock for the 20 trading days ending February 23, 2019) and the TSRU grant price ($21.03), plus dividend equivalents accrued during the life of the TSRU, divided by the settlement price, subject to the results being positive. |
| (6) | RSUs vest on the third anniversary of the grant date (February 23, 2015). Dividend equivalents are reinvested as additional RSUs during the restricted period. |
NOTE: The PSA and RSU values were converted to units using the closing stock price on February 21, 2012 of $21.22. The 5-Year TSRU values were converted to TSRUs using $4.12 and the 7-Year TSRU values were converted to TSRUs using $4.86, representing the estimated value at grant using the Monte Carlo Simulation model as of February 21, 2012.
The Committee customarily grants equity awards to eligible employees, including the NEOs, at its meeting held in late February of each year. Equity grants to certain newly hired employees, including executive officers, are effective on the last business day of the month of hire. Special equity grants to continuing employees are effective on the last business day of the month in which the award is approved. Stock option and TSRU grants have an exercise price equal to the closing market price of Pfizer’s common stock on their grant date. Our equity incentive plan prohibits the repricing or exchange of equity awards without shareholder approval.
| 58 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
| 2012 PROXY STATEMENT |

|
59 |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
Our pharmaceutical peer group for 2011 consisted of the following companies, which were selected based on their size and market capitalization and the complexity of their businesses, as well as the availability of comparative data. The Committee recognizes that while data are available on the performance of our non-U.S.-based peer companies, the compensation data are limited in terms of comparable benchmarks and other information as compared to peers based in the U.S.
2011 Pharmaceutical Peer Group
| Abbott Laboratories |
Johnson & Johnson | |
| Amgen |
Merck | |
| AstraZeneca |
Novartis | |
| Bristol-Myers Squibb |
Roche | |
| Eli Lilly |
Sanofi-Aventis | |
| GlaxoSmithKline |
Our general industry comparator group for 2011 was selected by the Committee from other industry sectors based on the same criteria as described above.
2011 General Industry Comparator Group
| Alcoa |
Honeywell | |
| Altria Group |
IBM | |
| Boeing |
Lockheed Martin | |
| Caterpillar |
PepsiCo | |
| Chevron |
Procter & Gamble | |
| Coca-Cola |
TimeWarner | |
| Comcast |
United Parcel Service | |
| Dell |
United Technologies | |
| Dow Chemical |
UnitedHealth Group | |
| DuPont |
Verizon | |
| FedEx |
Walt Disney | |
| General Electric |
The chart below compares Pfizer’s 2011 revenue, net income and market capitalization to the median revenue, net income and market capitalization for our pharmaceutical peer group and general industry comparator group.
| IN BILLIONS | PFIZER | PHARMACEUTICAL PEER GROUP MEDIAN |
GENERAL INDUSTRY MEDIAN |
|||||||||
| Revenue* |
$ | 67.4 | $ | 44.1 | $ | 58.2 | ||||||
| Reported Net Income* |
$ | 10.0 | $ | 7.8 | $ | 4.0 | ||||||
| Market Capitalization* |
$ | 164.2 | $ | 99.1 | $ | 73.8 | ||||||
| * | Revenue and Net Income based on published earnings releases. Market Capitalization as of February 22, 2012. |
Applying the Compensation Framework to Executive Positions
The Committee uses median compensation data for similar positions in both the pharmaceutical peer and general industry comparator groups, as well as an evaluation of internal equity among Pfizer executives, as a guide in setting compensation targets for each executive. Each compensation target is assigned a numbered salary grade to simplify the compensation administration process and help maintain internal equity.
Salary grades are used to determine the preliminary salary recommendation, target annual incentive award opportunity, and target long-term equity incentive award value for each executive position. Each salary grade is expressed as a range, with minimum, midpoint, and maximum salary level. Minimum and maximum salary range levels for each grade are set 25% below and above the salary range midpoint, which is intended to approximate the bottom and top quartiles for positions assigned to that grade. This framework provides a guide for the Committee’s determinations. The actual total compensation and/or amount of each compensation element for an individual executive may be more or less than this median.
| 60 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
| 2012 PROXY STATEMENT |

|
61 |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
The Company exceeded the target performance levels for 2011 set by the Committee for annual incentive purposes (see “Financial Results for Annual Incentive Purposes” earlier in this CD&A).
In addition to the corporate financial objectives, Mr. Read’s key accountabilities at the enterprise level included:
| •Improving The Performance of Our Innovative Core: Our adjusted research and development (R&D) spend for 2011 was reduced by nearly a billion dollars compared to 2010. The Company saw steady progress in our late stage pipeline, with over five approvals in the U.S. and E.U. We increased the focus of our R&D investments to our high-priority therapeutic areas including Cardiovascular, Metabolic and Neuroendocrine Diseases, Immunology and Inflammation, Neuroscience and Pain, Oncology and Vaccines. We advanced approximately 30 programs in our early- and mid-stage (Phase I and II) pipeline. To supplement internal efforts, we continued to invest in our R&D network and the capabilities designed to drive biomedical innovation.
•Maximizing Capital Allocation and Growth Opportunities: In 2011, we reduced our operating expenses and undertook several steps to maximize our capital allocation, such as completing the sale of Capsugel, completing the acquisitions of King and Ferrosan’s Consumer Health Business and several strategic deals, including out-licensing opportunities; conversely, we made data-driven decisions not to pursue a number of opportunities based on insufficient return-on-investment. We also completed a strategic review of the business which resulted in the ongoing exploration of strategic alternatives for our Animal Health and Nutrition businesses. We returned $15.2 billion in capital to shareholders through dividend payments and share repurchases. We achieved revenue growth in key branded assets, including Prevnar 13, Lyrica, Enbrel, and Sutent, as well as in Emerging Markets, particularly those where we increased our investments, such as China and Turkey.
•Reputation: We continued to improve our reputation in society through engagement with our customers, our shareholders, and the investor community. Our Chief Medical Officer, Dr. Freda Lewis-Hall, connected with customers via new channels to share health and medical information, especially in areas such as stroke prevention, smoking cessation and the early diagnosis of cancer. We continued to help qualified uninsured and underinsured patients access medicines for free or at a savings through the “Pfizer Helpful Answers” program in the U.S. Globally, we extended our commitment to help nations achieve the UN Millennium Goals, particularly in healthcare. To encourage a public policy environment that allows our innovation to serve patients today and in the future, we engaged government leaders on the value of our medicines and our contributions to the economy. Finally, we met with investors representing more than 30% of Pfizer’s ownership to apprise them of our ongoing activities and strategies.
•People and Culture: Based on colleague feedback and senior leader input, we evaluated how our culture needs to evolve to differentiate us from our competition. We designed a culture model to encourage ownership, collaboration and initiative; to build a strong, engaged leadership team; and to develop key talent. We also continued to develop diverse talent at senior levels and in the talent pipeline through new hires and promotions.
|
The Committee is responsible for evaluating Mr. Read’s performance against his objectives, with input from the other independent members of the Board, and for determining his compensation in consultation with the Committee’s independent consultant. In early 2012, each Board member (other than those elected in December 2011) completed a survey, on an anonymous basis, of their assessment of Mr. Read’s dealings with the Board and recommended areas of future focus. The Lead Independent Director and the Committee used the results of this survey and their assessment of Mr. Read’s performance against his objectives. The Committee used this information, as well as other key factors, to determine his compensation, which was ratified by the independent members of the Board.
PERFORMANCE OF OUR OTHER NAMED EXECUTIVE OFFICERS
The performance objectives for our other NEOs for 2011 included the corporate financial objectives noted above (50% weighting) and other objectives related to the achievement of individual financial, strategic and operational goals for their Business Units/Functions, as well as our imperative for Creating a Culture of Confidence and Trust, driven by initiative, collaboration and accountability, and developing our pipeline of talent.
| 62 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION DISCUSSION AND ANALYSIS
Mr. D’Amelio, Executive Vice President, Business Operations and Chief Financial Officer
| • | Met or exceeded all elements of 2011 financial guidance through careful collaboration and teamwork with the Company’s leadership team. |
| • | Generated $1.5 billion of operating cash flow incremental to 2011 operating plan through various finance and business operations initiatives. |
| • | Repurchased $9 billion in shares of Pfizer’s common stock, reducing the number of fully diluted weighted average shares by 2.5%. |
| • | Completed the divestiture of Capsugel on August 1, 2011, for $2.375 billion in cash. |
| • | Successfully closed the acquisition of King Pharmaceuticals, Inc. on February 28, 2011, and Ferrosan’s Consumer Health business on December 1, 2011. |
| • | Achieved the total cost reduction target associated with the Wyeth acquisition one year ahead of schedule. The total cost reduction target was approximately $4 to $5 billion, by the end of 2012, at 2008 average foreign exchange rates, in comparison with the 2008 pro-forma adjusted total costs of legacy Pfizer and legacy Wyeth operations. |
Dr. Dolsten, President, Worldwide Research and Development (WRD)
| • | Delivered four positive Proofs of Concept. |
| • | Achieved 13 Proof of Concept study starts. |
| • | Achieved 8 First in Human study starts. |
| • | Advanced 18 compounds into preclinical assessment. |
| • | Executed over 10 external deals to acquire compound licenses and technologies. |
| • | Supported on-time completion of key late-stage clinical programs and regulatory submissions, including dacomitinib Phase III study start, and Xalkori®, Eliquis® and tofacitinib submissions in the U.S. and Europe. |
| • | Expanded the Centers for Therapeutic Innovation to include 19 leading academic medical centers, such as Harvard University, Massachusetts General Hospital, University of California San Diego and Brigham and Women’s Hospital. |
Ms. Schulman, Executive Vice President and General Counsel; President and General Manager, Nutrition
| • | Protected Pfizer’s businesses, interests and products through ongoing counsel to the Board, its Committees and our management on a wide variety of complex legal and regulatory issues. |
| • | Continued to develop and implement comprehensive strategies to effectively manage and resolve litigation and claims against Pfizer. |
| • | Achieved continued success in managing legal costs through the enhancement and expansion of the Pfizer Legal Alliance—a highly innovative and widely-praised model developed and implemented for redefining the relationship between in-house and outside counsel resulting in the delivery of legal services with greater operational efficiency. |
| • | As head of our Nutrition business, led the business to over $2 billion in revenues, growing share while achieving greater operational efficiency and completing 32 new product launches. Achieved full-year revenue at 99.4% of plan and income before adjustments at 103.2% of plan. |
Mr. Simmons, President and General Manager, Emerging Markets and Established Products
| • | Achieved $17.79 billion in revenue for the Emerging Markets and Established Products Business Units (100% of budget) and income before adjustments of 101% of plan. |
– Launched new products in over 20 Emerging Market countries.
| • | Developed a strategic plan to accelerate growth in priority Emerging Markets, including Brazil, Russia, India, China, Mexico and Turkey, which resulted in: |
– Over 30 growth initiatives identified of which the near-term accretive initiatives were incorporated in 2012 operating plan.
| • | Achieved a leadership position in China. Pfizer is #1 in revenue and fastest growing company among the top ten biopharmaceutical companies in China. |
– Progressed geographic expansion in China and executed a memorandum of understanding with Hisun to participate in the generic segment.
| • | Successfully led the transition of Lipitor to the Established Products Division through innovative programs to retain the maximum value of the brand. |
| • | Quickly responded to drug shortages in the U.S., particularly in the Oncology area through nimble execution in the Established Products Division. |
| 2012 PROXY STATEMENT |

|
63 |
Table of Contents
The Executive Severance Plan became effective in February 2009 and provides for severance benefits to ELT members in the event of involuntary termination of employment without cause. Benefits under the Executive Severance Plan consist of cash severance equal to the greater of (a) one times pay (defined as base salary plus target annual incentive) or (b) 13 weeks pay plus three weeks pay per year of service, subject to a maximum of 104 weeks pay. In addition, eligible participants in the GPP receive a pro rata annual incentive for the year of termination, provided certain performance targets are achieved, as well as certain health and welfare benefits. Severance payments and benefits under the Executive Severance Plan are described in “Estimated Benefits Upon Termination” elsewhere in this Proxy Statement.
EMPLOYMENT AND RETIREMENT BENEFITS
We permit our executive officers to defer receipt of their earned annual incentives and any shares earned under PSAs. Certain of our NEOs are required to defer the receipt of RSUs (see “Other Compensation Policies – Tax Policies” below). Annual incentives may be deferred into either a Pfizer stock unit fund or a cash fund earning interest at 120% of the applicable federal long-term rate (which fluctuated between 3.16% and 5.05% in 2011). The Pfizer stock unit fund is credited with reinvested dividend equivalent units. PSAs may be deferred only into Pfizer common stock units. Legacy Wyeth employees (including Dr. Dolsten) were eligible to defer eligible compensation into the Wyeth Deferred Compensation Plan.
We provide a number of health and family security benefits, such as medical insurance, dental insurance, life insurance and long-term disability insurance. These benefits are available to all U.S.- and Puerto Rico-based employees, including the NEOs, and are comparable to those provided by the companies in the pharmaceutical and general industry comparator groups. These programs are designed to provide certain basic quality of life benefits and protections to Pfizer employees, including the NEOs, and at the same time enhance Pfizer’s attractiveness as an employer of choice. The Company’s annual cost of the benefits for each NEO ranges from approximately $13,000 to $25,000.
Pfizer maintains qualified defined benefit pension plans for the benefit of all its eligible U.S.- and Puerto Rico-based employees, including the NEOs, hired prior to January 1, 2011. For those U.S. employees earning in excess of the IRC limit ($245,000 for 2011), including the NEOs, Pfizer maintains related supplemental benefit restoration plans. The provisions and features of the qualified defined benefit pension plans and the related supplemental benefit restoration plans apply to all participants in those plans, including the NEOs. These plans are described in the narrative accompanying the “2011 Pension Benefits Table” and the “2011 Non-Qualified Deferred Compensation Table” below. Pfizer maintains savings plans that permit participants to make pre-tax, after-tax and/or Roth contributions of a portion of their eligible pay, up to certain limits. In addition, the Company maintains non-qualified savings plans that permit eligible participants to make pre-tax contributions in excess of tax law limitations on qualified plans. The Company provides matching contributions, which until December 31, 2011 varied by legacy company, with respect to employee contributions, up to certain limits. The provisions and features of the qualified savings plans and the related non-qualified supplemental savings plans apply to all participants in those plans, including the NEOs. A “legacy company” is the employee’s original employer, before Pfizer’s acquisitions of Warner-Lambert, Pharmacia and Wyeth, as applicable.
In addition to active employee benefits, we provide post-retirement medical and dental benefits to eligible retirees.
Effective January 1, 2010, all legacy Pfizer U.S. active employees (excluding legacy Wyeth U.S. active employees) who have at least 15 years of service after age 40 are eligible for post-retirement medical coverage. The value of the post-retirement medical coverage currently ranges from $123,000 to $275,000 over the course of retirement. Until December 31, 2011, legacy Wyeth U.S. active employees, including Dr. Dolsten, were eligible for post-retirement medical coverage under legacy Wyeth plan provisions that require employees to be at least age 55 with at least 10 years of service at retirement. Beginning January 1, 2012, legacy Wyeth U.S. active employees (including Dr. Dolsten) are eligible to participate in Pfizer’s post-retirement medical benefits if they were eligible under the legacy Wyeth requirements as of December 31, 2011 or reach the Pfizer requirements of at least 15 years of service after age 40.
| 64 |

|
2012 PROXY STATEMENT |
Table of Contents
We provide a limited number of perquisites (personal benefits) to our NEOs, including the limited use of company aircraft, financial counseling and home security services and, for the CEO, the use of a company car and driver. The transportation benefits provide increased efficiencies and allow more productive use of our executives’ time and, in turn, greater focus on Pfizer-related activities. We do not provide tax “gross-ups” for perquisites provided to ELT members, except in the case of certain relocation expenses (consistent with our relocation policy for U.S.-based employees generally); therefore, any taxes on perquisites (other than certain relocation expenses) are paid by the executives.
COMPANY AIRCRAFT
As a result of the recommendations contained in an independent, third-party security study, the Board has determined that the CEO must use Company-provided aircraft for all air travel, including personal travel, to the maximum extent practicable. The security study also recommends that the CEO’s spouse and dependent children use Company-provided aircraft when they accompany the CEO, to the maximum extent practicable. Travel by the CEO’s spouse or dependent children is generally considered personal use and is subject to taxation and disclosure.
Other ELT members (including the NEOs) may use Company aircraft for limited personal travel. Effective January 1, 2012, personal use by ELT members (including the NEOs) is permitted only with the prior approval of the CEO or his designees and is subject to other limitations. Effective January 1, 2011, travel by Messrs. Read and D’Amelio to attend meetings of the Boards of Directors of Kimberly-Clark Corporation and Humana Inc., respectively, is treated as business travel in view of the significant benefits to the Company of their service on those Boards.
The amounts disclosed in the “All Other Compensation” column in the 2011 Summary Compensation Table and in the table below have been valued based on the incremental costs to the Company for the personal use of Company aircraft. Incremental costs for personal use consist of the variable costs incurred by Pfizer to operate the aircraft for such use, including fuel costs; crew expenses, including travel, hotels and meals; in-flight catering; landing, parking and handling fees; communications expenses; certain trip-related maintenance; and other trip-related variable costs, as well as certain costs of any “deadhead” flights. Such costs do not include fixed or non-variable costs that would be incurred whether or not there was any personal use of the aircraft, such as crew salaries and benefits, insurance costs, aircraft purchase costs, depreciation, and scheduled maintenance.
To the extent required by tax regulations, amounts associated with personal use of corporate aircraft are imputed as income to ELT members, including the CEO. These amounts are not grossed up for taxes and are generally lower than the incremental costs associated with personal use of Company aircraft.
CAR AND DRIVER
The Company’s policy on the use of cars and drivers is as follows:
| • | cars and drivers are available to all ELT members for business reasons; |
| • | ELT members (other than the CEO, as discussed below) are required to reimburse the Company for personal use; |
| • | for security reasons, cars and drivers are available to the CEO for personal use (including commuting); and |
| • | spouse/partner travel is generally considered personal use, and the incremental cost of such travel must be reimbursed to the Company. |
Incremental cost to the Company is calculated as a portion of the cost of the annual lease, a portion of the cost of the driver, and fuel used.
The costs of personal use of a car and driver by the CEO need not be reimbursed, and the unreimbursed incremental cost to the Company of personal use of a car and driver by Mr. Read in 2011 is reflected in the table below and in the “All Other Compensation” column in the 2011 Summary Compensation Table. For tax purposes, the cost of the cars and fuel is imputed as income to the CEO and is not grossed up for taxes by the Company. Tax regulations provide that as a result of the recommendations contained in the independent, third-party security study referred to above, the cost of the drivers is not reportable as income to the CEO.
OTHER PERQUISITES
The Company provides a taxable allowance of up to $10,000 per year to our executive officers for financial counseling services, which may include tax preparation and estate planning services. We value this benefit based on the actual charges for the services.
Home security systems are available to the ELT members. The cost of any such systems is imputed as income to the recipients, as required.
| 2012 PROXY STATEMENT |

|
65 |
Table of Contents
PERQUISITES
The Company purchases season and other tickets to sporting, cultural and other events for use in connection with its business. On occasion, these tickets are provided to employees, including ELT members, and non-employee Directors for personal use. There is no incremental cost associated with such tickets or other items. In addition, ELT members and/or non-employee Directors may from time to time receive tickets or other items from third parties (subject to our policies on conflicts of interest). The Company does not provide or reimburse for country club memberships for any executive officers.
The following table summarizes the incremental value of perquisites for the NEOs in 2011.
2011 Incremental Cost of Perquisites Provided to Named Executive Officers
| NAME | AIRCRAFT USAGE ($) |
FINANCIAL COUNSELING ($) |
CAR USAGE ($) |
HOME SECURITY ($) |
OTHER ($)(2) |
TOTAL ($) |
||||||||||||||||||
| I. Read |
125,999 | – | 47,896 | – | 1,393 | 175,288 | ||||||||||||||||||
| F. D’Amelio(1) |
70,030 | 8,144 | – | – | 2,376 | 80,550 | ||||||||||||||||||
| M. Dolsten(1) |
44,375 | 13,426 | – | – | – | 57,801 | ||||||||||||||||||
| A. W. Schulman |
51,610 | – | – | 626 | 1,436 | 53,672 | ||||||||||||||||||
| D. Simmons |
54,805 | 6,500 | – | – | – | 61,305 | ||||||||||||||||||
| (1) | There is a $10,000 annual limit on financial counseling services provided to each NEO. The amounts shown for Mr. D’Amelio and Dr. Dolsten include $796 and $7,425, respectively, for financial counseling services provided in 2010 but paid in 2011. |
| (2) | The amounts shown represent certain personal benefits provided in association with business travel. |
| 66 |

|
2012 PROXY STATEMENT |
Table of Contents
| 2012 PROXY STATEMENT |

|
67 |
Table of Contents
| 68 |

|
2012 PROXY STATEMENT |
Table of Contents
POLICY—CRITERIA FOR SELECTION OF COMMITTEE CONSULTANT
The Committee has established the following criteria used to select a consultant to the Compensation Committee:
| • | Degree of independence |
– Financial independence—measured by dollar volume of other business conducted with Pfizer
– Independent thinking—subjectively assessed by their known work as well as information gathered in screening interviews
| • | Familiarity with the business environment |
– Knowledge of the pharmaceutical industry
– Specific knowledge of Pfizer, its senior management, and Board of Directors
– Broad knowledge of general industry current practices and emerging trends
– Public relations
| • | Particular strengths and/or distinguishing characteristics including, but not limited to: |
– Creative thinking
– Strong understanding of corporate governance
– Special areas of expertise
– Ability to establish rapport and dynamic presence with groups
| • | References from current clients where the consultant acts in an advisory role similar to the role desired by the Committee |
| • | Potential issues |
– Conflicts of interest with other clients or Committee members
– Degree of availability/accessibility
| 2012 PROXY STATEMENT |

|
69 |
Table of Contents
2011 Summary Compensation Table
| NAME AND PRINCIPAL POSITION |
YEAR | SALARY ($) |
BONUS(1) ($) |
STOCK ($) |
OPTION ($) |
NON-EQUITY ($) |
CHANGE IN QUALIFIED EARNINGS(5) ($) |
ALL OTHER ($) |
TOTAL ($) |
|||||||||||||||||||||||||||
| I. Read Chairman and Chief |
2011 | 1,700,000 | – | 5,684,218 | 6,916,435 | 3,500,000 | 6,893,407 | 319,288 | 25,013,348 | |||||||||||||||||||||||||||
| 2010 | 1,199,000 | – | 2,673,276 | 837,556 | 1,500,000 | 10,976,628 | 209,652 | 17,396,112 | ||||||||||||||||||||||||||||
| 2009 | 1,139,500 | 500,000 | 2,854,366 | 1,154,478 | 1,657,000 | 1,915,639 | 226,643 | 9,447,626 | ||||||||||||||||||||||||||||
|
F. D’Amelio EVP, Business Operations |
2011 | 1,200,000 | – | 2,046,310 | 1,832,194 | 1,440,000 | 984,814 | 187,425 | 7,690,743 | |||||||||||||||||||||||||||
| 2010 | 1,090,000 | – | 2,673,276 | 837,556 | 1,175,000 | 530,418 | 193,823 | 6,500,073 | ||||||||||||||||||||||||||||
| 2009 | 1,060,000 | 600,000 | 2,904,366 | 1,204,478 | 1,420,000 | 465,428 | 205,287 | 7,859,559 | ||||||||||||||||||||||||||||
| M. Dolsten(7) President, Worldwide Research and |
2011 | 1,100,000 | – | 2,046,310 | 1,832,194 | 1,490,000 | 417,430 | 90,801 | 6,976,735 | |||||||||||||||||||||||||||
| 2010 | 900,000 | 1,050,000 | 1,985,860 | 622,183 | 1,000,000 | 284,639 | 61,004 | 5,903,686 | ||||||||||||||||||||||||||||
|
A. W. Schulman(8) EVP, General Counsel, |
2011 | 900,000 | – | 1,705,287 | 1,526,834 | 1,190,000 | 348,369 | 94,172 | 5,764,662 | |||||||||||||||||||||||||||
| D. Simmons(8) President and GM, Emerging Markets and Established Products |
2011 | 850,000 | – | 1,591,598 | 1,425,043 | 1,135,000 | 843,070 | 132,180 | 5,976,891 | |||||||||||||||||||||||||||
| * | Mr. Read served as President and Chief Executive Officer from December 5, 2010 until his election as Chairman and Chief Executive Officer on December 12, 2011. |
| (1) | The amounts shown in this column for Messrs. Read and D’Amelio represent one-time cash incentive awards made in 2009 in recognition of their performance and leadership in connection with the successful completion of the Wyeth acquisition on October 15, 2009. In October 2009, Messrs. Read and D’Amelio received special one-time cash and equity incentive awards totaling $1.0 million and $1.2 million, respectively, in recognition of their performance and leadership in connection with the successful completion of the Wyeth acquisition on October 15, 2009, of which 50% was paid in cash. The amount shown for Dr. Dolsten in 2010 relates to sign-on cash incentive awards, totaling $2.1 million, under his employment offer. The awards were paid in two equal installments in 2009 and 2010. |
| (2) | The amounts shown in this column represent the grant date fair values for the RSU and PSA Awards granted in 2011, 2010 and 2009 and the STI Shift Awards granted in 2010 and 2009, including RSUs granted to Messrs. Read and D’Amelio in recognition of their performance and leadership in connection with the successful completion of the Wyeth acquisition on October 15, 2009. Further information regarding the 2011 awards is included in the “2011 Grants of Plan-Based Awards” and “2011 Outstanding Equity Awards at Fiscal Year-End” tables elsewhere in this Proxy Statement. The grant date fair values of the performance-based awards reflected in this column (PSAs granted in 2011, 2010 and 2009 and STI Shift Awards granted in 2010 and 2009) are the target payouts based on the probable outcome of the performance condition, determined as of the grant date. The maximum potential values of the 2011 PSAs would be as follows: Mr. Read—$6,365,795; Mr. D’Amelio—$2,291,676; Dr. Dolsten—$2,291,676; Ms. Schulman—$1,909,762; and Mr. Simmons—$1,782,442. Information related to the performance-based award program is included in “Performance Share Awards” elsewhere in this Proxy Statement. The grant date fair values have been determined based on the assumptions and methodologies set forth in the Company’s 2011 Financial Report (Note 13, Share-Based Payments). |
| (3) | The amounts shown in this column represent the grant date fair values of the TSRUs awarded in 2011, 2010 and 2009, respectively, including the TSRUs granted to Messrs. Read and D’Amelio in recognition of their performance and leadership in connection with the successful completion of the Wyeth acquisition on October 15, 2009. The grant date fair values have been determined based on the assumptions and methodologies set forth in the Company’s 2011 Financial Report (Note 13, Share-Based Payments). |
| (4) | The amounts shown in this column represent annual cash incentive awards made to the NEOs under the GPP. Further information regarding the 2011 awards is included in the “Annual Incentive Awards” table elsewhere in this Proxy Statement. |
| (5) | The Company does not pay “above market” interest on non-qualified deferred compensation to employees; therefore, this column reflects pension accruals only. The 2011 pension accrual amounts represent the difference between the December 31, 2011 and December 31, 2010 present values of age 65 accrued pensions, or the current benefit if the NEO is eligible for an unreduced pension under the Retirement Plan and supplemental retirement plan, based on the pension plan assumptions for each year, as shown in the footnotes to the “Pension Plan Assumptions” table later in this Proxy Statement. The 2011 and 2010 amounts for Mr. Read reflect his attainment of the “Rule of 90” (age plus service greater than or equal to 90) in November 2010. This provides him with an unreduced pension benefit upon his retirement. Further information regarding pension plans is included in the “2011 Pension Benefits Table” later in this Proxy Statement. |
| (6) | The amounts shown in this column represent the sum of the Company’s Savings Plan and Supplemental Savings Plan matching contributions and the incremental cost to the Company of perquisites received by the NEOs. The Savings Plan matching contributions include Company matching funds under the Pfizer Savings Plan (a tax-qualified retirement savings plan) and under the related Supplemental Savings Plan. These plans are discussed in more detail in the “2011 Non-Qualified Deferred Compensation” table later in this Proxy Statement. Certain 2009 amounts reflect minor adjustments to amounts previously reported. Additional information regarding 2011 perquisites is provided under “Perquisites” elsewhere in this Proxy Statement. |
| (7) | Dr. Dolsten joined Pfizer on the closing of the Wyeth transaction in October 2009 as President, Worldwide BioTherapeutics and was not an NEO in 2009. Under Dr. Dolsten’s employment offer, he received sign-on equity incentive awards valued at a total of $3.1 million. The equity incentive award of $1.0 million granted in the form of RSUs is payable on the third anniversary of the grant date (October 30, 2012). In addition, he received a one-time long-term incentive award advancement of $700,000, granted in the form of RSUs and payable on the third anniversary of the grant date (October 30, 2012) and is entitled to receive, upon retirement, a pension make-up equal to the difference between $49,728 per year and the respective total straight life pension plan annuity, to the extent applicable. |
| (8) | Ms. Schulman and Mr. Simmons were not NEOs for 2010 and 2009. |
| 70 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION TABLES
The following Grants of Plan-Based Awards Table provides additional information about non-equity incentive awards and long-term incentive awards granted to our NEOs during the year ended December 31, 2011. The long-term incentive awards were made under the 2004 Stock Plan, as amended and restated, and are described in the CD&A section headed “Elements of Executive Compensation.”
2011 Grants of Plan-Based Awards Table
| NAME (A) | GRANT DATE (B) |
ESTIMATED FUTURE PAYOUTS UNDER NON-EQUITY INCENTIVE PLAN AWARDS |
ESTIMATED FUTURE PAYOUTS UNDER EQUITY INCENTIVE PLAN AWARDS |
|||||||||||||||||||||||||||||||||||||||||
| THRESHOLD (C) |
TARGET (D) |
MAXIMUM ($) (E) |
THRESHOLD (#) (F) |
TARGET (#)/(1) (G) |
MAXIMUM (#) (H) |
ALL OTHER STOCK NUMBER OF STOCK (#) (I) |
ALL OTHER TSRU NUMBER OF (#) (J) |
EXERCISE OR BASE PRICE ($/Sh) (K) |
GRANT DATE FAIR VALUE OF STOCK AND TSRUs(2) ($) (L) |
|||||||||||||||||||||||||||||||||||
| I. Read(3) |
2/24/2011 | 584,112 | 18.90 | 2,570,093 | ||||||||||||||||||||||||||||||||||||||||
| 483,559 | 18.90 | 2,519,342 | ||||||||||||||||||||||||||||||||||||||||||
| 420,000 | 20.90 | 1,827,000 | ||||||||||||||||||||||||||||||||||||||||||
| 132,345 | 2,501,321 | |||||||||||||||||||||||||||||||||||||||||||
| 0 | 2,587,500 | 5,175,000 | 43,674 | (4) | 132,345 | (4) | 264,690 | (4) | 3,182,897 | |||||||||||||||||||||||||||||||||||
| F. D’Amelio |
2/24/2011 | 210,280 | 18.90 | 925,232 | ||||||||||||||||||||||||||||||||||||||||
| 174,081 | 18.90 | 906,962 | ||||||||||||||||||||||||||||||||||||||||||
| 47,644 | 900,472 | |||||||||||||||||||||||||||||||||||||||||||
| 0 | 1,125,000 | 2,250,000 | 15,723 | (4) | 47,644 | (4) | 95,288 | (4) | 1,145,838 | |||||||||||||||||||||||||||||||||||
| M. Dolsten |
2/24/2011 | 210,280 | 18.90 | 925,232 | ||||||||||||||||||||||||||||||||||||||||
| 174,081 | 18.90 | 906,962 | ||||||||||||||||||||||||||||||||||||||||||
| 47,644 | 900,472 | |||||||||||||||||||||||||||||||||||||||||||
| 0 | 1,125,000 | 2,250,000 | 15,723 | (4) | 47,644 | (4) | 95,288 | (4) | 1,145,838 | |||||||||||||||||||||||||||||||||||
| A. W. Schulman |
2/24/2011 | 175,234 | 18.90 | 771,030 | ||||||||||||||||||||||||||||||||||||||||
| 145,068 | 18.90 | 755,804 | ||||||||||||||||||||||||||||||||||||||||||
| 39,704 | 750,406 | |||||||||||||||||||||||||||||||||||||||||||
| 0 | 918,000 | 1,836,000 | 13,102 | (4) | 39,704 | (4) | 79,408 | (4) | 954,881 | |||||||||||||||||||||||||||||||||||
| D. Simmons |
2/24/2011 | 163,551 | 18.90 | 719,624 | ||||||||||||||||||||||||||||||||||||||||
| 135,397 | 18.90 | 705,418 | ||||||||||||||||||||||||||||||||||||||||||
| 37,057 | 700,377 | |||||||||||||||||||||||||||||||||||||||||||
| 0 | 918,000 | 1,836,000 | 12,229 | (4) | 37,057 | (4) | 74,114 | (4) | 891,221 | |||||||||||||||||||||||||||||||||||
| (1) | The PSA and RSU award values were converted to units using the closing stock price of $18.89 on February 22, 2011; the 5-Year, 7-Year and 7-Year (premium) TSRU values were converted using $4.28, $5.17 and $4.29, respectively, the estimated value using the Monte Carlo Simulation model as of February 22, 2011. See Note 3 for information regarding Premium-Priced 7-Year TSRUs. |
| (2) | The amounts shown in this column represent the award values as of the grant dates. The values of RSUs, PSAs, 5-Year, 7-Year and 7-Year (premium) TSRUs are shown at the respective fair values of $18.90, $24.05, $4.40, $5.21 and $4.35, as of February 24, 2011. See Note 3 for information regarding Premium-Priced 7-Year TSRUs. |
| (3) | The TSRU amounts includes 420,000 Premium-Priced 7-Year TSRUs with a grant price of $20.90, representing a 25% premium over the closing stock price on December 3, 2010, the last trading day prior to Mr. Read being elected President and CEO. Other than the grant price, all other terms of the award are identical to those of the annual grant described in the “Elements of Executive Compensation” table in the CD&A. |
| (4) | The amounts represent the threshold, target, and maximum share payouts under our Performance Share Award Program for the January 1, 2011—December 31, 2013 performance period. The payment for threshold performance ranges from 0% to 33% of target. |
| 2012 PROXY STATEMENT |

|
71 |
Table of Contents
COMPENSATION TABLES
The following table summarizes the equity awards we have made to our NEOs that were outstanding as of December 31, 2011.
2011 Outstanding Equity Awards at Fiscal Year-End Table
| NAME (A) | OPTION/SAR/TSRU AWARDS (2) | STOCK AWARDS (2) | ||||||||||||||||||||||||||||||||||||||||||
| GRANT DATE/ SHARE PERIOD (1) |
NUMBER OF (#) (B) |
NUMBER OF (#) (C) |
NUMBER OF (#)(B) |
NUMBER (#) (C) |
EQUITY (#)(D) |
OPTION/ ($) (E) |
OPTION/ (F) |
NUMBER (#) (G) |
MARKET ($) (H) |
EQUITY (#) (L) |
EQUITY ($) (J) |
|||||||||||||||||||||||||||||||||
| I. Read |
2/28/2002 | 100,000 | 41.30 | 2/27/2012 | ||||||||||||||||||||||||||||||||||||||||
| 2/27/2003 | 120,000 | 29.33 | 2/26/2013 | |||||||||||||||||||||||||||||||||||||||||
| 2/26/2004 | 140,000 | 37.15 | 2/25/2014 | |||||||||||||||||||||||||||||||||||||||||
| 2/24/2005 | 145,000 | 26.20 | 2/23/2015 | |||||||||||||||||||||||||||||||||||||||||
| 2/23/2006 | 193,000 | 26.20 | 2/22/2016 | |||||||||||||||||||||||||||||||||||||||||
| 2/22/2007 | 250,000 | 25.87 | 2/21/2017 | |||||||||||||||||||||||||||||||||||||||||
| 9/28/2007 | 25,000 | 24.43 | 9/27/2017 | |||||||||||||||||||||||||||||||||||||||||
| 2/28/2008 | 168,739 | 22.55 | 2/28/2013 | |||||||||||||||||||||||||||||||||||||||||
| 2/26/2009 | 223,881 | 12.70 | 2/26/2014 | 76,006 | 1,644,770 | |||||||||||||||||||||||||||||||||||||||
| 2/26/2009 | (3) | 58,188 | 1,259,188 | |||||||||||||||||||||||||||||||||||||||||
| 10/30/2009 | 54,585 | 17.03 | 10/30/2014 | 16,109 | 348,599 | |||||||||||||||||||||||||||||||||||||||
| 2/25/2010 | 197,072 | 17.69 | 2/25/2015 | 52,467 | 1,135,386 | |||||||||||||||||||||||||||||||||||||||
| 2/25/2010 | (4) | 38,975 | 843,419 | |||||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | 584,112 | 18.90 | 2/24/2016 | 136,490 | 2,953,644 | |||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | (5) | 483,559 | 18.90 | 2/24/2018 | 28,662 | 620,246 | ||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | (7) | 420,000 | 20.90 | 2/24/2018 | ||||||||||||||||||||||||||||||||||||||||
| 1/1/2009-12/31/2011 | 67,822 | 1,467,668 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2010-12/31/2012 | 48,747 | 1,054,885 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2011-12/31/2013 | 132,345 | 2,863,946 | ||||||||||||||||||||||||||||||||||||||||||
| F. D’Amelio |
9/28/2007 | 292,000 | 24.43 | 9/27/2017 | ||||||||||||||||||||||||||||||||||||||||
| 2/28/2008 | 168,739 | 22.55 | 2/28/2013 | |||||||||||||||||||||||||||||||||||||||||
| 2/26/2009 | 223,881 | 12.70 | 2/26/2014 | 76,006 | 1,644,770 | |||||||||||||||||||||||||||||||||||||||
| 2/26/2009 | (3) | 58,188 | 1,259,188 | |||||||||||||||||||||||||||||||||||||||||
| 10/30/2009 | 65,502 | 17.03 | 10/30/2014 | 19,331 | 418,323 | |||||||||||||||||||||||||||||||||||||||
| 2/25/2010 | 197,072 | 17.69 | 2/25/2015 | 52,467 | 1,135,386 | |||||||||||||||||||||||||||||||||||||||
| 2/25/2010 | (4) | 38,975 | 843,419 | |||||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | 210,280 | 18.90 | 2/24/2016 | 49,136 | 1,063,303 | |||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | (5) | 174,081 | 18.90 | 2/24/2018 | 28,662 | 620,246 | ||||||||||||||||||||||||||||||||||||||
| 1/1/2009-12/31/2011 | 67,822 | 1,467,668 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2010-12/31/2012 | 48,747 | 1,054,885 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2011-12/31/2013 | 47,644 | 1,031,016 | ||||||||||||||||||||||||||||||||||||||||||
| M. Dolsten |
10/30/2009 | 64,437 | 1,394,417 | |||||||||||||||||||||||||||||||||||||||||
| 10/30/2009 | 45,106 | 976,094 | ||||||||||||||||||||||||||||||||||||||||||
| 2/25/2010 | 146,396 | 17.69 | 2/25/2015 | 38,975 | 843,419 | |||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | 210,280 | 18.90 | 2/24/2016 | 49,136 | 1,063,303 | |||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | (5) | 174,081 | 18.90 | 2/24/2018 | 21,292 | 460,759 | ||||||||||||||||||||||||||||||||||||||
| 3/5/2009 | (6) | 3,000,100 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2010-12/31/2012 | 36,212 | 783,628 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2011-12/31/2013 | 47,644 | 1,031,016 | ||||||||||||||||||||||||||||||||||||||||||
|
A. W. Schulman |
6/30/2008 | 100,000 | 17.47 | 6/29/2018 | ||||||||||||||||||||||||||||||||||||||||
| 2/26/2009 | 111,940 | 12.70 | 2/26/2014 | 38,003 | 822,385 | |||||||||||||||||||||||||||||||||||||||
| 2/25/2010 | 84,459 | 17.69 | 2/25/2015 | 22,485 | 486,575 | |||||||||||||||||||||||||||||||||||||||
| 2/25/2010 | (4) | 16,489 | 356,822 | |||||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | 175,234 | 18.90 | 2/24/2016 | 40,947 | 886,093 | |||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | (5) | 145,068 | 18.90 | 2/24/2018 | 12,284 | 265,826 | ||||||||||||||||||||||||||||||||||||||
| 1/1/2009-12/31/2011 | 33,911 | 733,834 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2010-12/31/2012 | 20,891 | 452,081 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2011-12/31/2013 | 39,704 | 859,195 | ||||||||||||||||||||||||||||||||||||||||||
| 72 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION TABLES
| NAME (A) | OPTION/SAR/TSRU AWARDS (2) | STOCK AWARDS (2) | ||||||||||||||||||||||||||||||||||||||||||
| GRANT DATE/ SHARE PERIOD (1) |
NUMBER
OF (#) (B) |
NUMBER
OF (#) (C) |
NUMBER
OF (#)(B) |
NUMBER
OF (#) (C) |
EQUITY (#)(D) |
OPTION/ ($) (E) |
OPTION/ (F) |
NUMBER (#) (G) |
MARKET ($) (H) |
EQUITY (#) (L) |
EQUITY ($) (J) |
|||||||||||||||||||||||||||||||||
| D. Simmons |
2/28/2002 | 20,000 | 41.30 | 2/27/2012 | ||||||||||||||||||||||||||||||||||||||||
| 2/27/2003 | 21,000 | 29.33 | 2/26/2013 | |||||||||||||||||||||||||||||||||||||||||
| 2/26/2004 | 27,000 | 37.15 | 2/25/2014 | |||||||||||||||||||||||||||||||||||||||||
| 2/25/2005 | 16,500 | 26.20 | 2/23/2015 | |||||||||||||||||||||||||||||||||||||||||
| 2/23/2006 | 17,000 | 26.20 | 2/22/2016 | |||||||||||||||||||||||||||||||||||||||||
| 2/22/2007 | 43,000 | 25.87 | 2/21/2017 | |||||||||||||||||||||||||||||||||||||||||
| 2/28/2008 | 24,401 | 22.55 | 2/28/2013 | |||||||||||||||||||||||||||||||||||||||||
| 1/30/2009 | 11,448 | 247,735 | ||||||||||||||||||||||||||||||||||||||||||
| 2/26/2009 | 49,440 | 12.70 | 2/26/2014 | 16,784 | 363,206 | |||||||||||||||||||||||||||||||||||||||
| 2/26/2009 | (3) | 7,541 | 163,187 | |||||||||||||||||||||||||||||||||||||||||
| 12/31/2009 | 26,767 | 18.19 | 12/31/2014 | 7,470 | 161,651 | |||||||||||||||||||||||||||||||||||||||
| 1/29/2010 | 10,871 | 235,248 | ||||||||||||||||||||||||||||||||||||||||||
| 2/25/2010 | 67,568 | 17.69 | 2/25/2015 | 17,988 | 389,260 | |||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | 163,551 | 18.90 | 2/24/2016 | 38,217 | 827,016 | |||||||||||||||||||||||||||||||||||||||
| 2/24/2011 | 135,397 | 18.90 | 2/24/2018 | |||||||||||||||||||||||||||||||||||||||||
| 1/1/2009-12/31/2011 | 14,977 | 324,102 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2010-12/31/2012 | 16,713 | 361,669 | ||||||||||||||||||||||||||||||||||||||||||
| 1/1/2011-12/31/2013 | 37,057 | 801,913 | ||||||||||||||||||||||||||||||||||||||||||
| (1) | For better understanding of this table, we have included an additional column showing the grant date of stock options, SARs/TSRUs and RSUs and the associated performance period for the PSAs. |
| (2) | Stock options become exercisable in accordance with the vesting schedule below: |
| GRANT DATE | VESTING | |
| 2/28/2002 | 1/3 per year in years 3, 4 and 5 - Mr. Read | |
| 2/28/2002 | Full vesting after 3 years - Mr. Simmons | |
| 2/27/2003 | 1/3 per year in years 3, 4 and 5 - Mr. Read | |
| 2/27/2003 | Full vesting after 3 years - Mr. Simmons | |
| 2/26/2004 | 1/3 per year in years 3, 4 and 5 - Mr. Read | |
| 2/26/2004 | Full vesting after 3 years - Mr. Simmons | |
| 2/24/2005 | 1/3 per year in years 3, 4 and 5 - Mr. Read | |
| 2/25/2005 | Full vesting after 3 years - Mr. Simmons | |
| 2/23/2006 | Full vesting after 3 years | |
| 2/22/2007 | Full vesting after 3 years | |
| 9/28/2007 | 1/3 per year in years 1, 2 and 3 - Mr. D’Amelio | |
| 9/28/2007 | Full vesting after 3 years - Mr. Read | |
| 6/30/2008 | Full vesting after 3 years |
SARs/TSRUs vest and are settled in accordance with the schedule below:
| GRANT DATE | VESTING | |
| 2/28/2008 | Full vesting after 3 years and settled after 5 years | |
| 2/26/2009 | Full vesting after 3 years and settled after 5 years | |
| 10/30/2009 | Full vesting after 3 years and settled after 5 years | |
| 12/31/2009 | Full vesting after 3 years and settled after 5 years | |
| 2/25/2010 | Full vesting after 3 years and settled after 5 years | |
| 2/24/2011 | Full vesting after 3 years and settled after 5 years or 7 years |
| 2012 PROXY STATEMENT |

|
73 |
Table of Contents
COMPENSATION TABLES
RSUs vest in accordance with the schedule below:
| GRANT DATE | VESTING | |
| 1/30/2009 | Full vesting after 3 years | |
| 2/26/2009 | Full vesting after 3 years | |
| 10/30/2009 | Full vesting after 3 years | |
| 12/31/2009 | Full vesting after 3 years | |
| 1/29/2010 | Full vesting after 3 years | |
| 2/25/2010 | Full vesting after 3 years | |
| 2/24/2011 | Full vesting after 3 years |
| (3) | This RSU grant represents the portion paid from the 2008 STI Shift Award as RSUs, as elected by the executive. |
| (4) | This RSU grant represents the portion paid from the 2009 STI Shift Award as RSUs, as elected by the executive. |
| (5) | This RSU grant represents the portion paid from the 2010 STI Shift Award as RSUs, as elected by the executive. |
| (6) | This represents the 2009 Wyeth LTI Cash Award. This will be settled in cash 3 years following the vesting date. No interest is earned on these awards. |
| (7) | Mr. Read received Premium-Priced 7-Year TSRUs at a grant price of $20.90. |
The following Option Exercises and Stock Vested Table provides additional information about the value realized by the NEOs on option award exercises, stock/unit award vestings and the STI Shift Award payouts during the year ended December 31, 2011.
2011 Option Exercises and Stock Vested Table
| NAME | OPTION AWARDS |
RESTRICTED STOCK/RESTRICTED STOCK UNITS |
PERFORMANCE SHARES 2009-2011 PAID FEB 2012(1) |
STI SHIFT AWARD |
||||||||||||||||||||||||||||||||
| NUMBER OF SHARES EXERCISE |
VALUE EXERCISE |
NUMBER OF SHARES VESTING |
NUMBER OF SHARES |
VALUE REALIZED ON |
NUMBER OF SHARES VESTING |
NUMBER OF SHARES |
VALUE REALIZED |
VALUE REALIZED ($) |
||||||||||||||||||||||||||||
| I. Read |
– | – | 48,708 | – | (3) | 937,146 | 75,251 | 33,885 | 1,593,816 | 525,000 | ||||||||||||||||||||||||||
| F. D’Amelio |
– | – | 48,708 | 17,643 | 937,146 | 75,251 | 34,782 | 1,593,816 | 525,000 | |||||||||||||||||||||||||||
| M. Dolsten(2) |
– | – | – | – | – | – | – | – | 390,000 | |||||||||||||||||||||||||||
| A. W. Schulman |
– | – | 116,038 | 57,864 | 2,390,385 | 37,626 | 18,990 | 796,919 | 225,000 | |||||||||||||||||||||||||||
| D. Simmons |
– | – | 19,636 | 7,198 | 365,443 | 16,618 | 7,483 | 351,969 | 360,000 | |||||||||||||||||||||||||||
| (1) | The PSAs were determined based on relative TSR performance over the 2009-2011 performance period and were paid in February 2012. |
| (2) | Dr. Dolsten did not have any option exercises or stock vesting in 2011. |
| (3) | Due to IRC Section 162(m), which applies to our CEO and the other NEOs (excluding the CFO), when RSUs vest, the payment of shares is automatically deferred until the earlier of the time it can be reasonably expected that the NEO is no longer subject to Section 162(m) or the January 31st following termination of employment. |
| (4) | The RSUs vested on February 28, 2011 at $19.24 for Mr. D’Amelio; June 30, 2011 at $20.60 for Ms. Schulman; and January 31, 2011, February 23, 2011 and February 28, 2011 at $18.22, $18.76 and $19.24, respectively, for Mr. Simmons. Performance Shares vested on February 26, 2012 at $21.18. |
| (5) | The amounts shown in this column represent the 50% cash payout from the February 24, 2011 STI Shift Award paid in cash. The remaining 50% RSU award was reported in the “2011 Outstanding Equity Awards at Fiscal Year-End” table. Mr. Simmons was not an ELT member at the time of the grant. Pursuant to a prior election, he received 100% of the Award in cash. |
| 74 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION TABLES
The following 2011 Pension Benefits Table shows the present value of accumulated benefits payable to each of our NEOs under the Pfizer Consolidated Pension Plan (the “Retirement Plan”), which retains the pension formulas under the legacy plans, including the Pfizer Retirement Annuity Plan (the “PRAP”), the Wyeth Retirement Plan-United States (the “Wyeth Plan”) and the related non-funded legacy Pfizer Supplemental Retirement Plan (the “Supplemental Retirement Plan”), and the non-funded legacy Wyeth Supplemental Executive Retirement Plan (the “Wyeth Supplemental Retirement Plan”) (collectively, the “Supplemental Plans”).
| NAME | PLAN NAME | NUMBER OF (#) |
AGE 65 ($) |
PRESENT VALUE OF ACCUMULATED BENEFIT(1) ($) |
PAYMENTS ($) |
IMMEDIATE 12/31/2011 ($) |
LUMP ($) |
|||||||||||||||||||
| I. Read(3) |
Qualified Plan | 33 | 126,304 | 1,841,738 | – | 126,304 | 1,808,312 | |||||||||||||||||||
| Supplemental Plan | 1,725,828 | 25,552,739 | – | 1,725,828 | 24,709,006 | |||||||||||||||||||||
| F. D’Amelio |
Qualified Plan | 4 | 16,555 | 123,485 | – | – | – | |||||||||||||||||||
| Supplemental Plan(4) | 417,206 | 3,208,250 | – | – | – | |||||||||||||||||||||
| M. Dolsten(5) |
Qualified Plan | 3 | 15,606 | 117,344 | – | – | – | |||||||||||||||||||
| Supplemental Plan | 110,903 | 844,265 | – | – | – | |||||||||||||||||||||
| A. W. Schulman |
Qualified Plan | 3 | 13,647 | 89,168 | – | – | – | |||||||||||||||||||
| Supplemental Plan | 96,688 | 649,890 | – | – | – | |||||||||||||||||||||
| D. Simmons |
Qualified Plan | 15 | 57,248 | 307,936 | – | – | – | |||||||||||||||||||
| Supplemental Plan | 260,186 | 1,454,968 | – | – | – | |||||||||||||||||||||
| (1) | The present value of these benefits is based on the December 31, 2011 assumptions as shown below, used in determining our annual pension expense for fiscal 2012. |
| (2) | These amounts reflect the values of annuities if paid as a lump sum benefit as of January 1, 2012, as indicated above, only for NEOs eligible to retire as of that date. |
| (3) | The amount for Mr. Read reflects his attainment of the “Rule of 90” (age plus service greater than or equal to 90) in November 2010. This provides him with an unreduced pension benefit upon his retirement. |
| (4) | Under the terms of Mr. D’Amelio’s offer letter, he will receive an additional six years of benefit accrual service for pension purposes after he completes five years of service in 2012. The amounts shown above include $251,861 in the Supplemental Plan Age 65 Single-Life Annuity Payment and $1,936,772 in the Supplemental Plan Present Value of Accumulated Benefit, both of which are attributable to the additional six years of service. |
| (5) | The retirement benefits for Dr. Dolsten are based on the provisions of the Wyeth Retirement Plan formula of the Pfizer Consolidated Pension Plan and the Wyeth Supplemental Retirement Plan. Under the terms of Dr. Dolsten’s offer letter, Pfizer will provide a pension make-up equal to the difference between $49,728 per year and the respective total straight life pension plan annuity for Dr. Dolsten. |
The Retirement Plan
The Retirement Plan is a funded, tax-qualified, non-contributory defined benefit pension plan that covers certain employees, including the NEOs.
Retirement Plan (PRAP formula) and Supplemental Retirement Plan—Messrs. Read, D’Amelio and Simmons and Ms. Schulman
Benefits under the Retirement Plan (PRAP formula) are based on the employee’s years of service and highest average earnings for a five calendar-year period and are payable after retirement in the form of an annuity or a lump sum.
Benefits under the Retirement Plan are calculated as an annuity equal to the greater of:
| • | 1.4% of the employee’s highest final average earnings for a five-year calendar period multiplied by years of service; and |
| • | 1.75% of such earnings less 1.5% of the primary Social Security benefit multiplied by years of service. |
Years of service under these formulas cannot exceed 35.
Compensation covered by the Retirement Plan and the related Supplemental Plan for Messrs. Read, D’Amelio and Simmons and Ms. Schulman for 2011 equals the sum of the amounts set forth for 2011 in the “Salary” and “Non-Equity Incentive Plan Compensation” columns of the 2011 Summary Compensation Table. Mr. Read’s covered compensation also includes restricted stock awards granted on or prior to April 26, 2001 and any performance-based share awards granted for performance periods beginning before January 1, 2001. After the payment of the awards for the five-year period ended on December 31, 2004, no further performance-based share awards are included in the determination of pensions under the Retirement Plan or the Supplemental Retirement Plan.
| 2012 PROXY STATEMENT |

|
75 |
Table of Contents
COMPENSATION TABLES
Retirement Plan (Wyeth Plan formula) and Wyeth Supplemental Retirement Plan—Dr. Dolsten
Benefits under the Retirement Plan (Wyeth Plan formula) are based on the employee’s years of service and the employee’s final average pension earnings for the five-highest years of service within the last 10 years of service and are payable after retirement in the form of an annuity or a lump sum. Compensation covered under the Wyeth Plan formula and the related Wyeth Supplemental Plan for Dr. Dolsten for 2011 includes salary at April 1, 2011 and the bonus paid in 2011 for 2010 performance.
Benefits under the Retirement Plan (Wyeth Plan formula) are calculated as an annuity equal to 2% of the employee’s final average pension earnings, multiplied by years of credited service, less 1/60th of the annual primary Social Security benefit, multiplied by years of credited service.
Years of service under this formula cannot exceed 30.
General
Contributions to the Retirement Plan are made entirely by Pfizer and are paid into a trust fund from which benefits are paid.
The amount of annual earnings that may be considered in calculating benefits under the Retirement Plan is limited by law. For 2011, the annual limitation was $245,000. The Retirement Plan currently limits pensions paid under the Retirement Plans to an annual maximum of $195,000, payable at age 65 in accordance with IRC requirements. Under the Supplemental Plans, Pfizer provides, out of its general assets, amounts substantially equal to the difference between the amount that may be paid under the Retirement Plan and the amount that would be paid in the absence of these IRC limits. The Supplemental Plans are non-funded; however, in certain circumstances Pfizer has established and funded trusts to secure obligations to make payments under the Supplemental Plans.
Beginning January 1, 2012, all legacy Wyeth employees (including Dr. Dolsten) began earning benefits under the PRAP formula in the Retirement Plan and Supplemental Retirement Plan.
The present value of accumulated benefits has been computed based on the assumptions as of December 31, 2011 in the following table, which were used in developing our financial statement disclosures:
| ASSUMPTIONS AS OF | 12/31/2009 | 12/31/2010 | 12/31/2011 | |||
| Discount Rate |
6.30% for qualified pension plans, 6.20% for non-qualified pension plans |
5.90% for qualified pension plans, 5.80% for non-qualified pension plans | 5.10% for qualified pension plans, 5.00% for non-qualified pension plans | |||
| Lump Sum Interest Rate |
2.70% for annuity payments expected to be made during first 5 years; 6.00% for payments made between 5 and 20 years; and 6.80% for payments made after 20 years prior to reflecting the 5-year phase-in from GATT 30-year Treasury rate of 4.35% | 2.00% for annuity payments expected to be made during first 5 years; 5.20% for payments made between 5 and 20 years; and 6.50% for payments made after 20 years prior to reflecting the 5-year phase in from GATT 30-year Treasury rate of 4.40%. For legacy Wyeth 3.25% | 1.90% for annuity payments expected to be made during first 5 years; 4.30% for payments made between 5 and 20 years; and 5.10% for payments made after 20 years prior. For legacy Wyeth 3.25% | |||
| Percent Electing Lump Sum |
80%/70%(2) - Pfizer |
80%/70%(2} - Pfizer 85% - Wyeth |
80%/70%(2) - Pfizer 85% - Wyeth | |||
| Mortality Table for Lumps Sums |
Unisex mortality table specified by IRS Revenue Ruling 2007-67, based on RP 2000 table, with projected mortality improvements (7-15 years) | For legacy Pfizer, unisex mortality table specified by IRS Revenue Ruling 2007-67, based on RP 2000 table, with projected mortality improvements (7-15 years). For legacy Wyeth, Unisex 1994 Group Annuity Mortality Table, blended 50% Male and 50% Female | For legacy Pfizer, unisex mortality table specified by IRS Revenue Ruling 2007-67, based on RP 2000 table, with projected mortality improvements (7-15 years). For legacy Wyeth, Unisex 1994 Group Annuity Mortality Table blended 50% Male and 50% Female | |||
| Mortality Table for Annuities |
Separate annuitant and non-annuitant rates for the 2010 plan year, as set forth in regulation 1.412(l)(7)-1 | Separate annuitant and non-annuitant rates for the 2011 plan year, as set forth in regulation 1.412(l)(7)-1 | Separate annuitant and non-annuitant rates for the 2012 plan year, as set forth in regulation 1.412(l)(7)-1 |
| (1) | These assumptions are also used to determine the change in pension value in the 2011 Summary Compensation Table. |
| (2) | 80% relates to the Qualified Plan and 70% relates to the Supplemental Plan. Only applies to the extent the executive is eligible to receive a lump sum. |
We have included an additional column titled “Age 65 Single-Life Annuity Payment” in the 2011 Pension Benefits Table. The amounts listed in this column represent the amount payable to the executive upon attaining age 65, assuming retirement. We have also added a column showing the immediately payable pension benefit as well as a column showing the lump sum value of that benefit for those NEOs who meet the retirement criteria under the Plans.
| 76 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION TABLES
Early Retirement Provisions
Under the Retirement Plan and Supplemental Retirement Plans, the normal retirement age is 65. Under the Retirement Plan (PRAP formula), if a participant terminates employment with an age and years of service combination equal to or greater than 90, the employee is entitled to receive either an annuity or a lump sum that is unreduced under the terms of the Retirement Plan or the Supplemental Retirement Plan for early payment. Mr. Read attained this milestone during 2010. If an employee retires on or after age 55 with 10 or more years of service, that participant may elect to receive either an early retirement annuity payment reduced by 4% per year (prorated for partial years) for each year between benefit commencement and age 65, or a lump sum payment. If an employee does not satisfy any of the above criteria and has three years of vesting service under the Retirement Plan, that participant may elect to receive an annuity starting on or after age 55, which is reduced by 6% per year for each year (prorated for partial years) prior to age 65; a lump sum payment is not available.
Under the Retirement Plan (Wyeth Plan formula) and the Wyeth Supplemental Retirement Plan, the normal retirement age is 65. If an employee retires on or after age 55 with 10 or more years of vesting service, the employee may elect to receive an early retirement benefit, reduced by 3% per year (prorated for partial years) for each year between benefit commencement and age 65. If an employee does not satisfy the above criteria, and has at least 3 but less than 10 years of vesting service under the Retirement Plan, that employee may elect to receive his or her benefit on or after age 55, when the benefit is reduced by the actuarial present value (on average 6% per year) for each year between benefit commencement and age 65.
Beginning January 1, 2012, all legacy Wyeth employees (including Dr. Dolsten) began earning benefits under the Retirement Plan and Supplemental Retirement Plan according to the Pfizer PRAP formula.
Board Policy on Pension Benefits for Executives
The Board has adopted a policy providing that it will seek shareholder approval prior to the payment of amounts to any senior executive from the Company’s defined benefit pension plans if his or her benefit, computed as a single life annuity, will exceed 100% of the senior executive’s final average salary, as calculated at the discretion of the Committee. This policy applies to all benefit accruals after January 1, 2006. For purposes of this policy, “final average salary” means the average of the highest five calendar years’ earnings (as defined by the Committee and not based on the legacy pension plan definition), where earning includes salary earned during the year and annual cash incentives (or bonus) earned for the year.
| 2012 PROXY STATEMENT |

|
77 |
Table of Contents
COMPENSATION TABLES
The following Non-Qualified Deferred Compensation Table summarizes activity during 2011 and account balances in our various non-qualified savings and deferral plans for our NEOs. The following plans and programs permit the executives to defer amounts previously earned on a pre-tax basis: Pfizer Non-Funded Deferred Compensation and Supplemental Savings Plan (“PSSP”), Global Performance Plan (“GPP”), Performance Share Awards (“PSA”), Short-Term Incentive Shift Award (“STI Shift Award”), Wyeth Supplemental Employee Savings Plan (“Wyeth SESP”) and Wyeth Deferred Compensation Plan (“Wyeth DCP”). The PSSP and Wyeth SESP are non-qualified supplemental savings plans that provide for the deferral of compensation that otherwise could have been deferred under the related tax-qualified 401(k) plans but for the application of certain IRC limitations and for Company matching contributions based on the executive’s contributions. Other than the matching contributions (and the earnings thereon) in the PSSP, the account balances in these plans are generally attributable to deferrals of previously earned compensation and the earnings on those amounts.
2011 Non-Qualified Deferred Compensation Table(1)
| NAME | PLAN(2) | EXECUTIVE CONTRIBUTIONS IN 2011 ($) |
PFIZER CONTRIBUTIONS IN 2011 ($) |
AGGREGATE EARNINGS IN 2011 ($) |
AGGREGATE WITHDRAWALS/ DISTRIBUTIONS ($) |
AGGREGATE BALANCE AT 12/31/11 ($) |
||||||||||||||||
| I. Read |
PSSP | 177,300 | 132,975 | 464,988 | – | 2,284,573 | ||||||||||||||||
| Deferred GPP | – | – | – | – | – | |||||||||||||||||
| Deferred PSA | – | – | 852,076 | – | 3,819,375 | |||||||||||||||||
| Deferred RSU(3) | 937,146 | – | 372,899 | – | 2,089,489 | |||||||||||||||||
| Total: | 1,114,446 | 132,975 | 1,689,963 | – | 8,193,437 | |||||||||||||||||
| F. D’Amelio |
PSSP | 127,800 | 95,850 | 52,887 | – | 931,507 | ||||||||||||||||
| Deferred GPP | – | – | – | – | – | |||||||||||||||||
| Deferred PSA | – | – | – | – | – | |||||||||||||||||
| Total: | 127,800 | 95,850 | 52,887 | – | 931,507 | |||||||||||||||||
| M. Dolsten |
Wyeth SESP | 51,300 | 25,650 | 3,161 | – | 163,933 | ||||||||||||||||
| Deferred GPP | – | – | – | – | – | |||||||||||||||||
| Total: | 51,300 | 25,650 | 3,161 | – | 163,933 | |||||||||||||||||
| A. W. Schulman |
PSSP | 131,000 | 29,475 | 18,261 | – | 363,967 | ||||||||||||||||
| Deferred GPP | – | – | – | – | – | |||||||||||||||||
| Deferred STI Shift Award | – | – | 12,728 | – | 298,361 | |||||||||||||||||
| Total: | 131,000 | 29,475 | 30,989 | – | 662,328 | |||||||||||||||||
| D. Simmons |
PSSP | 104,667 | 59,850 | 58,686 | – | 748,237 | ||||||||||||||||
| Deferred GPP | – | – | 21,825 | – | 97,887 | |||||||||||||||||
| Deferred STI Shift Award | – | – | 10,580 | – | 67,973 | |||||||||||||||||
| Total: | 104,667 | 59,850 | 91,091 | – | 914,097 | |||||||||||||||||
| (1) | Contribution amounts reflected in this table are and have been reflected in the 2011 Summary Compensation Table and prior years’ summary compensation tables, as applicable. Aggregate earnings are not reflected in the summary compensation tables. |
| (2) | The PSSP contributions were based on the executive’s deferral election and the salary shown in the 2011 Summary Compensation Table, as well as annual incentive awards paid in 2011, previously reported. The Wyeth SESP contributions were based on the executive’s deferral election and the salary shown in the 2011 Summary Compensation Table. |
| (3) | Represents an RSU award vested on February 28, 2011 that was deferred due to IRC Section 162(m). Further information regarding the RSU vesting is reported in the 2011 Option Exercises and Stock Vested Table. |
Pfizer Savings Plans
The Company provides the Pfizer Savings Plan (the “Savings Plan”) to U.S.-based employees of the Company and the Pfizer Supplemental Savings Plan (the “PSSP”) to employees who meet the eligibility requirements. Legacy Wyeth executives (including Dr. Dolsten) are covered under the Wyeth Supplemental Employee Savings Plan (the “Wyeth SESP”). The Wyeth Savings Plan was merged into the Pfizer Savings Plan effective October 1, 2010, but the Wyeth matching formula was retained through December 31, 2011. Contribution amounts are reflected in the 2011 Summary Compensation Table or prior years’ summary compensation tables, as applicable. Earnings have not been included. These plans are described below.
The Savings Plan is a tax-qualified retirement savings plan. Participating employees may contribute up to 20% of “regular earnings” on a before-tax basis, Roth 401(k) basis and after-tax basis, into their Savings Plan accounts. “Regular earnings” for the Savings Plan include both salary and bonus or annual incentive awards. In addition, under the Savings Plan, we generally match an amount equal to one dollar for each dollar contributed by participating employees on the first 3% of their regular earnings, and fifty cents for each additional dollar contributed on the next 3% of their regular earnings. Matching contributions generally are invested in our common stock. Plan participants have the ability to immediately diversify the matching contribution investments.
| 78 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION TABLES
Dr. Dolsten was subject to a legacy Wyeth plan formula under which he could contribute up to 50% of base salary on a before-tax or after-tax basis. Under the plan, Dr. Dolsten received a 3% match if he contributed 6% or more of base pay, as defined under the legacy Wyeth plan. Matching contributions generally are invested in accordance with the instructions provided by the employee.
Beginning January 1, 2012, all legacy Wyeth employees (including Dr. Dolsten) began earning benefits under the Pfizer plan formula of the Savings Plan. In addition, for eligible legacy Wyeth employees (including Dr. Dolsten), participation in the Wyeth SESP was frozen on December 31, 2011, and beginning January 1, 2012 eligible legacy Wyeth employees (including Dr. Dolsten) began participating in the PSSP.
Pursuant to tax law limitations, effective for 2011, the Savings Plan limits the “additions” that can be made to a participating employee’s account to $49,000 per year. “Additions” include our matching contributions, before-tax contributions, Roth 401(k) contributions and after-tax contributions.
The tax law limits the amounts that may be allocated to tax-qualified savings plans and the amount of compensation that can be taken into account in computing benefits under the Savings Plan. The 2011 maximum before-tax and Roth 401(k) contribution limit was $16,500 per year (or $22,000 per year for eligible participants age 50 and over). In addition, no more than $245,000 of annual compensation may be taken into account in computing benefits under the Savings Plan.
The PSSP and Wyeth SESP are intended to pay, out of the general assets of the Company, an amount substantially equal to the difference between the amount that would have been allocated to an employee’s account as before-tax contributions, our matching contributions and the amount actually allocated under the Savings Plan if the limits described in the preceding paragraph did not exist. Under the PSSP, participants can elect to defer up to 20% of eligible wages on a before-tax basis. Under the Wyeth SESP, participants can elect to defer up to 6% of the eligible wages on a before-tax basis. Generally, under the PSSP, participants can elect to receive payments as a lump sum or in one to twenty annual installments following termination from service. Participants who do not make an election receive lump sum payments. Generally, under the Wyeth SESP, participants can elect to receive payments as a lump sum, or under limited circumstances, can elect to roll over their balances into the Wyeth Deferred Compensation Plan. In certain circumstances, we have established and funded trusts to secure our obligations to make payments under the PSSP and Wyeth SESP.
Amounts deferred, if any, under the PSSP or Wyeth SESP by the NEOs for 2011 are included in the “Salary” and “Non-Equity Incentive Plan Compensation” columns of the 2011 Summary Compensation Table. In the Non-Qualified Deferred Compensation table, PSSP/Wyeth SESP values are shown for each NEO. Executive contributions reflect the percent of salary and bonus the executive has elected to defer under the PSSP/Wyeth SESP. This matching contribution is shown in the “Pfizer Contributions” column of the table. For the NEOs, the Company’s matching contributions under the Savings Plan and the PSSP/Wyeth SESP are shown in the “All Other Compensation” column of the 2011 Summary Compensation Table. The “Aggregate Earnings” column in the above table represents the amount by which the PSSP/Wyeth SESP balance changed in the past fiscal year, net of employee and employer contributions.
ESTIMATED BENEFITS UPON TERMINATION
The following table shows the estimated benefits payable upon a hypothetical termination of employment under the Executive Severance Plan and under various termination scenarios as of December 31, 2011.
Estimated Benefits Upon Various Termination Scenarios
| NAME |
SEVERANCE(1) (A) ($) |
OTHER(2) (B) ($) |
TERMINATION WITHOUT CAUSE | TERMINATION ON CHANGE IN CONTROL |
DEATH OR DISABILITY |
|||||||||||||||||||||||
| LONG-TERM AWARD PAYOUTS(3) (C) ($) |
TOTAL (A+B+C) ($) |
LONG-TERM AWARD PAYOUTS(4) (D) ($) |
TOTAL (A+B+D) ($) |
LONG-TERM AWARD PAYOUTS(5) ($) |
||||||||||||||||||||||||
| I. Read |
8,575,000 | 14,843 | 13,895,724 | 22,485,567 | 24,006,339 | 32,596,182 | 24,006,339 | |||||||||||||||||||||
| F. D’Amelio |
2,325,000 | 19,043 | 11,251,162 | 13,595,205 | 16,647,970 | 18,992,013 | 16,647,970 | |||||||||||||||||||||
| M. Dolsten |
2,225,000 | 18,493 | 7,275,758 | 9,519,251 | 11,610,983 | 13,854,476 | 11,610,983 | |||||||||||||||||||||
|
A. W. Schulman |
1,818,000 | 23,846 | 4,425,946 | 6,267,792 | 7,604,071 | 9,445,917 | 7,604,071 | |||||||||||||||||||||
| D. Simmons |
1,972,000 | 22,071 | 3,504,152 | 5,498,223 | 6,368,901 | 8,362,972 | 6,368,901 | |||||||||||||||||||||
| (1) | These amounts represent severance equal to the greater of: (a) one year’s pay (defined as base salary and target bonus) or (b) 13 weeks pay plus 3 weeks pay per year of service, subject to a maximum of 104 weeks. These amounts do not include payments, if any, under the GPP. However, the individual would receive a pro rata portion of his or her targeted award under the GPP. |
| (2) | These amounts represent the Company cost of 12 months of active employee medical and life insurance coverage. |
| (3) | These amounts represent the value of long-term incentive awards which vest on termination of employment without cause using our closing stock price of $21.64 on December 31, 2011. |
| (4) | These amounts represent the value of long-term incentive awards which vest following a change in control using our closing stock price of $21.64 on December 31, 2011. |
| (5) | These amounts represent the value of long-term incentive awards which vest on termination of employment due to death or disability using our closing stock price of $21.64 on December 31, 2011. |
| 2012 PROXY STATEMENT |

|
79 |
Table of Contents
COMPENSATION TABLES
The NEOs are eligible for the following potential payments upon death, disability, retirement and a change in control, as described below:
Payments Made Upon Disability
Under our legacy Pfizer flexible benefits program, eligible employees, including the NEOs other than Dr. Dolsten, are provided with Company-paid long-term disability coverage of 50% of total pay, and may buy an increased level of coverage of up to 70% of total pay, subject to a $500,000 annual benefit limit. Through December 31, 2011, if the employee was vested in the Retirement Plan, those benefits continued to accrue while receiving disability benefits and health and life insurance coverage is continued. Beginning January 1, 2012, health and life insurance benefits will be provided for 24 months and Retirement Plan benefits will not continue to accrue to those who begin to receive long-term disability benefits. Under the legacy Wyeth health and insurance program, eligible employees, including Dr. Dolsten, have the ability to purchase long-term disability coverage of up to 60% of base pay, subject to a $360,000 annual benefit limit and health and life insurance coverage is continued for 24 months. Beginning January 1, 2012, all colleagues (including legacy Wyeth colleagues, including Dr. Dolsten) are covered under the Pfizer benefit plans.
Under the Long-Term Incentive Program, in the event of disability, PSAs are paid out at target; RSUs are paid in full; SARs/TSRUs vest and are settled on the fifth or seventh anniversary of the date of grant; and outstanding stock options continue to vest and become exercisable for the full option term, provided the executive remains permanently and totally disabled.
Payments Made Upon Death
Under our legacy Pfizer flexible benefits program, eligible employees, including the NEOs other than Dr. Dolsten, have the ability to purchase life insurance benefits of eight times pay (subject to evidence of insurability requirements) up to a maximum of $4.0 million. Pfizer provides one times base pay with a maximum cap of $2.0 million paid by the Company. The deceased executive’s pension and deferred compensation are also payable in accordance with the plans and the executive’s election.
Under our legacy Wyeth health and insurance benefits program, eligible employees, including Dr. Dolsten, have the ability to purchase up to eight times base pay in life insurance benefits with no limit. In addition, coverage of one times base pay, with no maximum in coverage, is paid by the Company. The deceased executive’s pension and other retirement savings and deferred compensation are also payable in accordance with the plans and the executive’s election. Beginning January 1, 2012, all colleagues (including legacy Wyeth colleagues, including Dr. Dolsten) are covered under the Pfizer benefit plans.
Under the Long-Term Incentive Program, in the event of death, PSAs are paid out at target; RSUs are paid in full; SARs/TSRUs vest and are immediately settled; and outstanding stock options are exercisable for the remainder of the option term if the participant is eligible for retirement; if not, the stock options remain exercisable for up to two years.
Payments Made Upon Retirement
Under the Long-Term Incentive Program, if a participant retires (after attaining age 55 with at least 10 years of service) after the first anniversary of the grant date, RSUs are prorated based on service subsequent to the grant date; SARs/TSRUs continue to vest and are settled on the fifth or seventh anniversary of the grant date; and outstanding stock options are exercisable for the full term of the option. PSAs are prorated at the end of the performance period if the participant is employed through December 31 of the year of grant. If the retirement takes place prior to the first anniversary of the grant date, these long-term awards are forfeited. Based on age and years of service, Mr. Read is the only NEO eligible for retirement treatment and would receive $10,496,662 under his long-term awards as of December 31, 2011 in the event of his retirement.
See “Retirement and Savings Plans” and “Retiree Health Care Benefits” for further information on health care, retirement and savings plan benefits under Pfizer’s plans.
Payments Made Upon Change in Control
Under the Long-Term Incentive Program, if a participant’s employment is terminated within 24 months of a change in control, PSAs are paid out at target; RSUs are paid in full; unvested SARs/TSRUs vest and are immediately settled; vested SARs/TSRUs are settled on the fifth or seventh anniversary of the date of grant; and outstanding stock options are exercisable for the remainder of the option term.
This table provides certain information as of December 31, 2011 with respect to our equity compensation plans:
Equity Compensation Plan Information
| PLAN CATEGORY | (A) NUMBER OF SECURITIES |
(B) WEIGHTED-AVERAGE |
(C) NUMBER OF SECURITIES |
|||||||||
| Equity compensation plans approved by security holders |
477,049,686 | (1) | $ | 24.61 | 318,948,604 | (2) | ||||||
| Equity compensation plans not approved by security holders |
0 | N/A | 0 | |||||||||
| Total |
477,049,686 | $ | 24.61 | 318,948,604 | ||||||||
| 80 |

|
2012 PROXY STATEMENT |
Table of Contents
COMPENSATION TABLES
| (1) | This amount includes the following: |
| • | 406,954,758 shares issuable upon the exercise of outstanding stock options with a weighted average exercise price of $24.96. |
| • | 6,092,590 shares issuable pursuant to outstanding share awards that have been granted under the Pfizer Inc. 2004 Stock Plan, as amended and restated (the “2004 Stock Plan”), but not yet earned as of December 31, 2011. The number of shares, if any, to be issued pursuant to such outstanding awards will be determined by a formula that measures our performance, in terms of total shareholder return, over the applicable performance period relative to the performance of the pharmaceutical peer group, as discussed above. Since these awards have no exercise price, they are not included in the weighted average exercise price calculation in column (b). |
| • | 41,940,120 shares subject to restricted stock units, granted under the 2004 Stock Plan. Since these awards have no exercise price, they are not included in the weighted average exercise price calculation in column (b). |
| • | 19,165,851 non-vested shares and 2,896,367 vested shares pursuant to TSRUs granted under the 2004 Stock Plan with a weighted average exercise price of $18.06. The number of shares, if any, to be issued pursuant to outstanding TSRUs will be determined by the difference between the defined settlement price and the grant price, plus the dividends accumulated during the respective 5- and 7-year terms. The settlement price is the 20-day average closing stock price ending on the fifth or seventh anniversary of the grant, as applicable. |
| (2) | This amount represents the number of shares available (318,948,604) for issuance pursuant to stock options and awards that could be granted in the future under the 2004 Stock Plan. Under the 2004 Stock Plan, any option granted reduces the available number of shares on a one-to-one basis and any whole share award granted reduces the available number of shares on a two-to-one basis. |
In 2003, Pfizer acquired Pharmacia Corporation and assumed various stock-based plans. No further grants may be made under any of these plans. As of December 31, 2011, under the Pharmacia 2001 Long-Term Incentive Plan, 21,540,171 shares were issuable upon the exercise of outstanding stock options at a weighted average exercise price of $31.63. In addition, under the other assumed Pharmacia plans, as of December 31, 2011, there were 1,058,343 shares issuable upon the exercise of outstanding stock options, and those options had a weighted average exercise price per share of $27.51. Information regarding these options is not included in the above table.
In 2000, Pfizer acquired Warner-Lambert Company and assumed 217,653 shares issuable pursuant to the Warner-Lambert 1996 Stock Plan in settlement of Warner-Lambert directors’ compensation that had been deferred by certain former Warner-Lambert directors prior to Pfizer’s acquisition of Warner-Lambert. Information regarding those shares is not included in the above table.
On October 15, 2009, Pfizer acquired Wyeth and assumed the Wyeth Management Incentive Plan (the “MIP Plan”), pursuant to which no subsequent awards have been or will be made. As of December 31, 2011 there were 114,186 shares issuable in settlement of the participants’ accounts, which will be delivered upon separation from Pfizer, subject to meeting the requirements of the MIP Plan. Information regarding these shares is not included in the above table.
The following table contains reconciliations of 2011 and 2010 U.S. GAAP revenues and U.S. GAAP diluted EPS to revenues and adjusted diluted EPS for annual incentive purposes relating to the Financial Performance Table within this Proxy Statement (Unaudited).
Financial Measures
| (Billions, except per common share data) | 2011 | 2010 | ||||||
| Revenues |
$ | 67.4 | $ | 67.1 | ||||
| Foreign exchange impact relative to rates in effect for budget purposes |
(0.4 | ) | 0.3 | |||||
| Revenues for Annual Incentive purposes |
$ | 67.0 | $ | 67.4 | ||||
| Diluted EPS* |
$ | 1.27 | $ | 1.02 | ||||
| Purchase accounting adjustments—net of tax |
0.64 | 0.76 | ||||||
| Acquisition-related costs—net of tax |
0.19 | 0.36 | ||||||
| Discontinued operations—net of tax |
(0.17 | ) | (0.01 | ) | ||||
| Certain significant items—net of tax |
0.39 | 0.09 | ||||||
| Adjusted diluted EPS* |
$ | 2.31 | $ | 2.22 | ||||
| Foreign exchange impact relative to rates in effect for budget purposes |
(0.02 | ) | 0.04 | |||||
| Exclusion of non-recurring items |
(0.02 | ) | – | |||||
| Adjusted diluted EPS for Annual Incentive purposes |
$ | 2.27 | $ | 2.26 | ||||
| * | For a full reconciliation of adjusted diluted EPS, see the 2011 Financial Report. EPS amounts may not add due to rounding. |
| 2012 PROXY STATEMENT |

|
81 |
Table of Contents
Requirements, Including Deadlines, for Submission of Proxy Proposals and Nomination of Directors
Under the rules of the SEC, if a shareholder wants us to include a proposal in our Proxy Statement and form of proxy for presentation at our 2013 Annual Meeting of Shareholders, the proposal must be received by us at our principal executive offices at 235 East 42nd Street, New York, NY 10017-5755 by November 15, 2012. The proposal should be sent to the attention of the Secretary of the Company.
Under our By-laws, a shareholder must follow certain procedures to nominate persons for election as Directors or to introduce an item of business at an Annual Meeting of Shareholders. These procedures provide that nominations for Director nominees and/or an item of business to be introduced at an Annual Meeting of Shareholders must be submitted in writing to the Secretary of the Company at our principal executive offices. We must receive written notice of your intention to introduce a nomination or to propose an item of business at our 2013 Annual Meeting:
| • | if the 2013 Annual Meeting is being held within 25 days before or after the anniversary of the date of this year’s Annual Meeting (April 26, 2012), not less than 90 days nor more than 120 days in advance of the anniversary of the 2012 Annual Meeting; or |
| • | 10 days following the date on which notice of the date of the 2013 Annual Meeting is mailed or the public disclosure of the date of the 2013 Annual Meeting is made, whichever first occurs. |
For any other meeting, the nomination or item of business must be received by the tenth day following the date of public disclosure of the date of the meeting.
Our Annual Meeting of Shareholders is generally held on the fourth Thursday of April. Assuming that our 2013 Annual Meeting is held on schedule, to be “timely” within the meaning of Rule 14a-4(c) under the Securities Exchange Act of 1934, we must receive written notice of your intention to introduce a nomination or other item of business at that Meeting between December 27, 2012 and January 26, 2013. If we do not receive written notice during that time period, or if we meet certain other requirements of the SEC rules, the persons named as proxies in the proxy materials relating to that Meeting will use their discretion in voting the proxies when any such matters are raised at the Meeting.
The nomination must contain the following information about the nominee (amongst other information, as specified in the By-laws):
| • | name; |
| • | age; |
| • | business and residence addresses; |
| • | principal occupation or employment; |
| • | the class and number of shares of Pfizer stock owned (beneficially and of record) by the nominee; |
| • | the information that would be required under the rules of the SEC in a proxy statement or other filing required to be made in connection with the solicitation of proxies for election of directors pursuant to Section 14 of the Securities Exchange Act of 1934, and the rules and regulations promulgated thereunder; and |
| • | a signed consent of the nominee to serve as a Director of the Company, if elected. |
Notice of a proposed item of business must include (amongst other information, as specified in the By-laws):
| • | a brief description of the substance of, and the reasons for conducting, such business at such Meeting; and |
| • | as to the shareholder proponent and the beneficial owner, if any, on whose behalf the proposal is being made: |
| • | the name and address of each such person and of any holder of record of the shareholder proponent’s shares as they appear on our records; |
| • | the class and number of all shares of Pfizer stock owned by each such person (beneficially and of record) (with supporting documentation where appropriate); |
| • | any material interest of each such person, or any affiliates or associates of each such person, in such business; and |
| • | any other information relating to each such person that would be required to be disclosed in a proxy statement or other filing required to be made in connection with the solicitation of proxies by each such person with respect to the proposed business to be brought by each such person before the annual meeting pursuant to Section 14 of the Securities Exchange Act of 1934, and the rules and regulations promulgated thereunder. |
| 82 |

|
2012 PROXY STATEMENT |
Table of Contents
The Board is not aware of any matters that are expected to come before the 2012 Annual Meeting other than those referred to in this Proxy Statement. If any other matter should come before the Annual Meeting, the Proxy Committee intends to vote the proxies in accordance with its best judgment.
The Chairman of the Meeting may refuse to allow the transaction of any business, or to acknowledge the nomination of any person, not made in compliance with the procedures described above under “Requirements, including Deadlines, for Submission of Proxy Proposals and Nomination of Directors.”
Whether or not you plan to attend the Meeting, please vote by telephone, on the Internet, or by mail.
If you vote by telephone, the call is toll-free within the U.S., U.S. territories and Canada. No postage is required for mailing in the United States if you vote by mail using the enclosed prepaid envelope.
| 2012 PROXY STATEMENT |

|
83 |
Table of Contents
PFIZER INC.
CORPORATE GOVERNANCE PRINCIPLES
Role and Composition of the Board of Directors
1. General. The Board of Directors, which is elected by the shareholders, is the ultimate decision-making body of the Company, except with respect to those matters reserved to the shareholders. It selects the Chief Executive Officer and other members of the senior management team, which is charged with the conduct of the Company’s business. Having selected the senior management team, the Board acts as an advisor and counselor to senior management and ultimately monitors its performance. The function of the Board to monitor the performance of senior management is facilitated by the presence of non-employee Directors of stature who have substantive knowledge of the Company’s business.
2. Succession Planning. The Board also plans for succession to the position of Chief Executive Officer as well as certain other senior management positions. To assist the Board, the Chief Executive Officer annually provides the Board with an assessment of senior managers and their potential to succeed him or her. He or she also provides the Board with an assessment of persons considered potential successors to certain senior management positions.
3. Board Leadership. The independent Directors will annually elect a Chairman of the Board, who may or may not be the Chief Executive Officer of the Company. If the individual elected as Chairman of the Board is the Chief Executive Officer, the independent Directors shall also elect a Lead Independent Director. The Chairman of the Board shall preside at all meetings of the shareholders and of the Board as a whole, as well as over executive sessions of the independent Directors, and shall perform such other duties, and exercise such powers, as from time to time shall be prescribed in the Company’s By-laws or by the Board of Directors; provided that the Lead Independent Director, if any, shall preside over executive sessions of the Company’s independent Directors. In addition, the Lead Independent Director, if any, shall facilitate information flow and communication among the Directors and perform such other duties as may be specified by the Board and outlined in the Charter of the Lead Independent Director.
4. Director Independence. It is the policy of the Company that the Board consist of a majority of independent Directors. The Corporate Governance Committee of the Board has established Director Qualification Standards to assist it in determining Director independence, which either meet or exceed the independence requirements of the New York Stock Exchange (“NYSE”) corporate governance listing standards. The Board will consider all relevant facts and circumstances in making an independence determination, and not merely from the standpoint of the Director, but also from that of persons or organizations with which the Director has an affiliation.
5. Board Size. It is the policy of the Company that the number of Directors not exceed a number that can function efficiently as a body. The Corporate Governance Committee considers and makes recommendations to the Board concerning the appropriate size and needs of the Board. The Corporate Governance Committee considers candidates to fill new positions created by expansion and vacancies that occur by resignation, by retirement or for any other reason.
6. Selection Criteria. Candidates are selected for, among other things, their integrity, independence, diversity of experience, leadership and their ability to exercise sound judgment. Scientific expertise, prior government service and experience at policy-making levels involving issues affecting business, government, education, technology, as well as areas relevant to the Company’s global business, are among the most significant criteria. Final approval of a candidate is determined by the full Board.
7. Voting for Directors. In accordance with the Corporation’s By-laws, unless the Secretary of the Company determines that the number of nominees exceeds the number of Directors to be elected as of the record date for any meeting of the shareholders, a nominee must receive more votes cast for than against his or her election or re-election in order to be elected or re-elected to the Board. The Board expects a Director to tender his or her resignation if he or she fails to receive the required number of votes for re-election. The Board shall nominate for election or re-election as Director only candidates who agree to tender, promptly following such person’s failure to receive the required vote for election or re-election at the next meeting at which such person would face election or re-election, an irrevocable resignation that will be effective upon Board acceptance of such resignation. In addition, the Board shall fill Director vacancies and new directorships only with candidates who agree to tender, promptly following their appointment to the Board, the same form of resignation tendered by other Directors in accordance with this Corporate Governance Principle.
If an incumbent Director fails to receive the required vote for re-election, then, within 90 days following certification of the shareholder vote, the Corporate Governance Committee will act to determine whether to accept the Director’s resignation and will submit such recommendation for prompt consideration by the Board, and the Board will act on the Committee’s recommendation. The Corporate Governance Committee and the Board may consider any factors they deem relevant in deciding whether to accept a Director’s resignation.
Any Director who tenders his or her resignation pursuant to this provision shall not participate in the Corporate Governance Committee recommendation or Board action regarding whether to accept the resignation offer.
| i |
Table of Contents
Thereafter, the Board will promptly disclose its decision-making process and decision regarding whether to accept the Director’s resignation offer (or the reason(s) for rejecting the resignation offer, if applicable) in a Form 8-K furnished to the Securities and Exchange Commission.
If each member of the Corporate Governance Committee fails to receive the required vote in favor of his or her election in the same election, then those independent Directors who did receive the required vote shall appoint a committee amongst themselves to consider the resignation offers and recommend to the Board whether to accept them.
However, if the only Directors who receive the required vote in the same election constitute three or fewer Directors, all Directors may participate in the action regarding whether to accept the resignation offers.
8. Director Service on Other Public Boards. Ordinarily, Directors should not serve on more than four other boards of public companies in addition to the Company’s Board. Current positions in excess of these limits may be maintained unless the Board of Directors determines that doing so would impair the Director’s service on the Company’s Board.
9. Former Chief Executive Officer as Director. Effective 2001, upon retirement from the Company, the former Chief Executive Officer will not retain Board membership.
10. Change in Director Occupation. When a Director’s principal occupation or business association changes substantially during his or her tenure as a Director, that Director shall tender his or her resignation for consideration by the Corporate Governance Committee. The Corporate Governance Committee will recommend to the Board the action, if any, to be taken with respect to the resignation.
11. Director Compensation. The Corporate Governance Committee annually reviews the compensation of Directors.
12. Ownership Requirement. Each non-employee Director is required to hold at least $550,000 worth of Pfizer stock while serving as a Director of the Company. A Director’s holdings include units granted to the Director as compensation for Board service and shares or units held under a deferral or similar plan. A Director will have five years from the date of (a) his or her first election as a Director or (b) if later, an increase in the amount of Pfizer stock required to be held, to satisfy this ownership requirement
13. Director Retirement. Directors are required to retire from the Board when they reach the age of 73; a Director elected to the Board prior to his or her 73rd birthday may continue to serve until the annual shareholders meeting coincident with or next following his or her 73rd birthday. On the recommendation of the Corporate Governance Committee, the Board may waive this requirement as to any Director if it deems such waiver to be in the best interests of the Company.
14. Board and Committee Self-Evaluation. The Board, and each Committee, are required to conduct a self-evaluation of their performance at least annually.
15. Term Limits. The Board does not endorse arbitrary term limits on Directors’ service, nor does it believe in automatic annual re-nomination until Directors reach the mandatory retirement age. The Board self-evaluation process is an important determinant for continuing service.
16. Committees. It is the general policy of the Company that all major decisions be considered by the Board as a whole. As a consequence, the Committee structure of the Board is limited to those Committees considered to be basic to, or required or appropriate for, the operation of the Company. Currently these Committees are the Executive Committee, Audit Committee, Compensation Committee, Corporate Governance Committee, Regulatory and Compliance Committee and Science and Technology Committee.
The members and chairs of these Committees are recommended to the Board by the Corporate Governance Committee. The Audit Committee, Compensation Committee and Corporate Governance Committee are made up of only independent Directors. The membership of these Committees is rotated from time to time. In addition to the requirement that a majority of the Board satisfy the independence standards noted above in Paragraph 4, Director Independence, members of the Audit Committee also must satisfy an additional NYSE independence standard. Specifically, they may not accept directly or indirectly any consulting, advisory or other compensatory fee from Pfizer or any of its subsidiaries other than their Director compensation. As a matter of policy, the Board also will apply a separate and heightened independence standard to members of both the Compensation and Corporate Governance Committees. No member of either Committee may be a partner, member or principal of a law firm, accounting firm or investment banking firm that accepts consulting or advisory fees from Pfizer or any of its subsidiaries.
17. Director Orientation and Continuing Education. In furtherance of its policy of having major decisions made by the Board as a whole, the Company has a full orientation and continuing education process for Board members that includes extensive materials, meetings with key management and visits to Company facilities.
18. Chief Executive Officer Performance Goals and Annual Evaluation. The Compensation Committee is responsible for setting annual and long-term performance goals for the Chief Executive Officer and for evaluating his or her performance against such goals. The Committee meets annually with the Chief Executive Officer to receive his or her recommendations concerning such goals. Both the goals and the evaluation are then submitted for consideration by the independent Directors at a meeting or executive session of that group. The Committee then meets with the Chief Executive Officer to evaluate his or her performance against such goals.
19. Senior Management Performance Goals. The Compensation Committee also is responsible for setting annual and long-term performance goals and compensation for the direct reports to the Chief Executive Officer. These decisions are approved or ratified by action of the independent Directors at a meeting or executive session of that group.
| ii |
Table of Contents
20. Communication with Stakeholders. The Chief Executive Officer is responsible for establishing effective communications with the Company’s stakeholder groups, i.e., shareholders, customers, Company associates, communities, suppliers, creditors, governments and corporate partners.
It is the policy of the Company that management speaks for the Company. This policy does not preclude non-employee Directors, including the Chairman of the Board or the Lead Independent Director, from meeting with shareholders, but it is suggested that in most circumstances any such meetings be held with management present.
21. Annual Meeting Attendance. All Board members are expected to attend our Annual Meeting of Shareholders unless an emergency prevents them from doing so.
Board Functions
22. Agenda. The Chief Executive Officer, with approval from the Chairman of the Board or the Lead Independent Director, shall set the agenda for Board meetings with the understanding that the Board is responsible for providing suggestions for agenda items that are aligned with the advisory and monitoring functions of the Board. Agenda items that fall within the scope of responsibilities of a Board Committee are reviewed with the chair of that Committee. Any member of the Board may request that an item be included on the agenda.
23. Board Materials. Board materials related to agenda items are provided to Board members sufficiently in advance of Board meetings to allow the Directors to prepare for discussion of the items at the meeting.
24. Board Meetings. At the invitation of the Board, members of senior management recommended by the Chief Executive Officer shall attend Board meetings or portions thereof for the purpose of participating in discussions. Generally, presentations of matters to be considered by the Board are made by the manager responsible for that area of the Company’s operations.
25. Director Access to Corporate and Independent Advisors. In addition, Board members have free access to all other members of management and employees of the Company and, as necessary and appropriate, Board members may consult with independent legal, financial, accounting and other advisors to assist in their duties to the Company and its shareholders.
26. Executive Sessions. Executive sessions or meetings of non-employee Directors without management present are held regularly (at least four times a year) to review the report of the independent registered public accounting firm, the criteria upon which the performance of the Chief Executive Officer and other senior managers is based, the performance of the Chief Executive Officer against such criteria, the compensation of the Chief Executive Officer and other senior managers, and any other relevant matters. Meetings are held from time to time with the Chief Executive Officer for a general discussion of relevant subjects.
27. Annual Board Self-Evaluation. The Board, under the direction of the Corporate Governance Committee, will prepare an annual performance self-evaluation.
Committee Functions
28. Independence. The Audit, Compensation and Corporate Governance Committees consist only of independent Directors. A majority of the members of the Regulatory and Compliance Committee must be independent Directors.
29. Meeting Conduct. The frequency, length and agenda of meetings of each of the Committees are determined by the chair of the Committee. Sufficient time to consider the agenda items is provided. Materials related to agenda items are provided to the Committee members sufficiently in advance of the meeting where necessary to allow the members to prepare for discussion of the items at the meeting.
30. Scope of Responsibilities. The responsibilities of each of the Committees are determined by the Board from time to time.
31. Annual Committee Self-Evaluation. Each Committee is responsible for preparing an annual performance self-evaluation.
Policy on Poison Pills
32. Expiration of Rights Agreement. The Board amended Pfizer’s Rights Agreement, or “Poison Pill,” to cause the Agreement to expire on December 31, 2003. The term Poison Pill refers to a type of shareholder rights plan that some companies adopt to provide an opportunity for negotiation during a hostile takeover attempt.
The Board has adopted a statement of policy that it shall seek and obtain shareholder approval before adopting a Poison Pill; provided, however, that the Board may determine to act on its own to adopt a Poison Pill, if, under the circumstances, the Board, including the majority of the independent members of the Board, in its exercise of its fiduciary responsibilities, deems it to be in the best interest of Pfizer’s shareholders to adopt a Poison Pill without the delay in adoption that would come from the time reasonably anticipated to seek shareholder approval.
If the Board were ever to adopt a Poison Pill without prior shareholder approval, the Board would either submit the Poison Pill to shareholders for ratification, or would cause the Poison Pill to expire within one year.
The Corporate Governance Committee will review this Poison Pill policy statement on an annual basis, including the stipulation which addresses the Board’s fiduciary responsibility to act in the best interest of the shareholders without prior shareholder approval, and report to the Board any recommendations it may have concerning the policy.
Periodic Review of Corporate Governance Principles
33. These principles are reviewed by the Board at least annually.
| iii |
Table of Contents
Westin Governor Morris
2 Whippany Road
Morristown, New Jersey 07960
From Newark Liberty International Airport:
| • | Exit the airport and follow signs for I-78 West and stay on for approximately 8.5 miles |
| • | Take to Exit 48 for Route 24 |
| • | Stay on Route 24 West for approximately 9 miles |
| • | Take to Exit 1A for Whippany Road towards Morristown |
| • | Travel for approximately 1 mile to the hotel |
| • | The hotel will be on the right |
From Points North:
| • | Take I-287 South to Exit 37 (Route 24 East) |
| • | Follow Route 24 East to Exit 2A (Columbia Turnpike) |
| • | Exit the ramp and continue through 5 traffic lights |
| • | The hotel will be on the left |
From Points South:
| • | Take I-287 North to Exit 36A (Morris Avenue) |
| • | Stay in the right lane, and the road will bear to the right. Immediately after that curve, take the first left (Lindsay Drive) |
| • | The hotel will be on the left |
From Points East:
| • | Travel West on Route 24 and take Exit 2A to Route 510 (Columbia Turnpike) |
| • | Go straight through the first traffic light and continue to the end of Columbia Turnpike |
| • | Go straight through the light to the hotel entrance on the left |
From Points West:
| • | Take I-78 East to I-287 North |
| • | Exit 36A (Morris Avenue) and bear right |
| • | Turn left onto Lindsay Avenue |
| • | The hotel will be on the left |
From Route 80 East/West, George Washington Bridge, NE Pennsylvania:
| • | Follow to Exit 43. Choose I-287 South |
| • | Follow Signs for I-287 (South-Exit 37) Route 24 East |
| • | Follow to the first Exit 2A, Columbia Turnpike |
| • | Follow straight through the next 5 traffic lights and the hotel will be on the left side |
Table of Contents

Table of Contents

. MMMMMMMMMMMM Admission Ticket MMMMMMMMMMMMMMM C123456789 IMPORTANT ANNUAL MEETING INFORMATION 000004 000000000.000000 ext 000000000.000000 ext ENDORSEMENT_LINE SACKPACK 000000000.000000 ext 000000000.000000 ext MMMMMMMMM 000000000.000000 ext 000000000.000000 ext MR A SAMPLE Electronic Voting Instructions DESIGNATION (IF ANY) Available 24 hours a day, 7 days a week ADD 1 Instead of mailing your proxy, you may choose one of the voting ADD 2 methods outlined below to vote. ADD 3 ADD 4 VALIDATION DETAILS ARE LOCATED BELOW IN THE TITLE BAR. ADD 5 Proxies submitted by the Internet or telephone must be received by ADD 6 7:30 a.m., Eastern Daylight Time, on April 26, 2012. Vote by Internet • Go to www.investorvote.com/PFE • Or scan the QR code with your smartphone. • Follow the steps outlined on the secure website. Vote by telephone • Within the USA, US territories & Canada, call toll free 1-800-652-VOTE (8683) on a touch tone telephone. There is NO CHARGE to you for the call. • Outside the USA, US territories & Canada, call 1-781-575-2300 on a touch Using a black ink pen, mark your votes with an X as shown in X tone telephone. Standard rates will apply. this example. Please do not write outside the designated areas. • Follow the instructions provided by the recorded message. Annual Meeting Proxy Card 1234 5678 9012 345 3 IF YOU HAVE NOT VOTED VIA THE INTERNET OR TELEPHONE, FOLD ALONG THE PERFORATION, DETACH AND RETURN THE BOTTOM PORTION IN THE ENCLOSED ENVELOPE. 3 A The Board of Directors recommends votes “FOR” the listed nominees and “FOR” Proposals 2 and 3. 1. Election of Directors: For Against Abstain For Against Abstain For Against Abstain + 01—Dennis A. Ausiello 06—Helen H. Hobbs 11—Suzanne Nora Johnson 02—M. Anthony Burns 07—Constance J. Horner 12—Ian C. Read 03—W. Don Cornwell 08—James M. Kilts 13—Stephen W. Sanger 04—Frances D. Fergusson 09—George A. Lorch 14—Marc Tessier-Lavigne 05—William H. Gray, III 10—John P. Mascotte For Against Abstain For Against Abstain 2. Ratify the selection of KPMG LLP as independent registered 3. Advisory approval of executive compensation public accounting firm for 2012 The Board of Directors recommends a vote “AGAINST” Proposals 4-7. For Against Abstain 4. Shareholder proposal regarding publication of political contributions 5. Shareholder proposal regarding action by written consent 6. Shareholder proposal regarding special shareholder meetings 7. Shareholder proposal regarding advisory vote on director pay MMMMMMMC 1234567890 J N T MR A SAMPLE (THIS AREA IS SET UP TO ACCOMMODATE 140 CHARACTERS) MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND 1 U P X 1 3 1 2 2 2 1 MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND + 002CS40029 01F0AM
Table of Contents

. Admission Ticket 2012 Annual Meeting of Pfizer Inc. Shareholders Thursday, April 26, 2012 8:30 a.m., Eastern Daylight Time The Westin Governor Morris 2 Whippany Road Morristown, NJ 07960 Shareholders will be admitted to the Annual Meeting beginning at 8:00 a.m. Eastern Daylight Time. If you wish to attend, please plan to arrive early since seating will be limited. Please see the inside back cover of the Proxy Statement for directions. If you plan to attend the Annual Meeting, please bring this admission ticket with you. VOTE YOUR PFIZER SHARES Dear Pfizer Shareholder: Your vote is important! We encourage you to vote promptly and to take advantage of Internet or telephone voting, both of which are available 24 hours a day, seven days a week until 7:30 a.m., Eastern Daylight Time, on April 26, 2012. (If you vote on the Internet or by telephone, you do not need to mail your proxy card.) Receive Future Proxy Materials Electronically Help us make a positive difference to our environment by eliminating paper proxy mailings. With your consent, we will send all future proxy materials to you by e-mail, along with a link to the Pfizer proxy voting site. To register for electronic delivery of future proxy materials, go to www.computershare-na.com/green and follow the prompts. 3 IF YOU HAVE NOT VOTED VIA THE INTERNET OR TELEPHONE, FOLD ALONG THE PERFORATION, DETACH AND RETURN THE BOTTOM PORTION IN THE ENCLOSED ENVELOPE. 3 2012 Annual Meeting of Shareholders Proxy Solicited by the Board of Directors + The undersigned appoints Ian C. Read, Amy W. Schulman and Matthew Lepore, and each of them, as proxies, each with full power of substitution, and authorizes them to represent and to vote, as designated on the reverse side of this form, all the shares of common stock of Pfizer Inc. held of record by the undersigned on February 28, 2012, and all of the shares as to which the undersigned then had the right to give voting instructions to the record holder under the Pfizer Inc. Shareholder Investment Program, the Pfizer Savings Plan, or any other employee benefit plan or trust in which shares are held, at the Annual Meeting of Shareholders to be held on April 26, 2012 at 8:30 a.m., Eastern Daylight Time, at The Westin Governor Morris, 2 Whippany Road, Morristown, NJ 07960, or any adjournment or postponement of the Meeting. IF NO OTHER INDICATION IS MADE ON THE REVERSE SIDE OF THIS FORM, THE PROXIES WILL VOTE (AND ANY VOTING INSTRUCTIONS TO RECORD HOLDERS WILL BE GIVEN) FOR PROPOSALS 1, 2 AND 3, AND AGAINST PROPOSALS 4-7 AND, IN THEIR DISCRETION, UPON SUCH OTHER BUSINESS AS PROPERLY COMES BEFORE THE MEETING. B Non-Voting Items Change of Address — Please print your new address below. Comments — Please print your comments below. Meeting Attendance Mark the box to the right if you plan to attend the Annual Meeting. C Authorized Signatures — This section must be completed for your vote to be counted. — Date and Sign Below Please sign exactly as name(s) appear(s) hereon. Joint owners should each sign. When signing as attorney, executor, administrator, corporate officer, trustee, guardian, or custodian, please give full title. Date (mm/dd/yyyy) — Please print date below. Signature 1 — Please keep signature within the box. Signature 2 — Please keep signature within the box. IF VOTING BY MAIL, YOU MUST COMPLETE AND SIGN SECTION C ABOVE. +
Table of Contents

. NNNNNNNNNNNN + C 1234567890 IMPORTANT ANNUAL MEETING INFORMATION 000004 NNNNNN ENDORSEMENT_LINE SACKPACK NNNNNNNNN MR A SAMPLE DESIGNATION (IF ANY) ADD 1 ADD 2 ADD 3 ADD 4 ADD 5 ADD 6 Vote by Internet • Go to www.investorvote.com/PFE • Or scan the QR code with your smartphone • Follow the steps outlined on the secure website Annual Meeting Notice & Admission Ticket 1234 5678 9012 345 Important Notice Regarding the Availability of Proxy Materials for the 2012 Annual Meeting of Pfizer Inc. Shareholders to be held on April 26, 2012 Under Securities and Exchange Commission rules, you are receiving this notice that the proxy materials for the annual meeting are available on the Internet. Follow the instructions below to view the materials and vote online or request a copy. The items to be voted on and location of the annual meeting are on the reverse side. Your vote is important! This communication presents only an overview of the more complete proxy materials that are available to you on the Internet. We encourage you to access and review all of the important information contained in the proxy materials www.investorvote.com/PFE When you go online to view materials, you can also vote your shares. before voting. These materials are available at: ??Easy Online Access — A Convenient Way to View Proxy Materials and Vote Step 1: Go to www.investorvote.com/PFE. Step 2: Click on the icon on the right to view current meeting materials. Step 3: Return to the investorvote.com window and follow the instructions on the screen to log in. Step 4: Make your selection as instructed on each screen to select delivery preferences and vote. When you go online, you can also help the environment by consenting to receive electronic delivery of future materials. Obtaining a Copy of the Proxy Materials – If you want to receive a copy of these documents, you must request one. There is no charge to you for requesting a copy. Please make your request for a copy as instructed on the reverse side on or before April 12, 2012 to facilitate timely delivery. C O Y + 01F7DJ
Table of Contents

. Annual Meeting Notice & Admission Ticket 2012 Annual Meeting of Pfizer Inc. Shareholders Thursday, April 26, 2012 8:30 a.m., Eastern Daylight Time The Westin Governor Morris 2 Whippany Road Morristown, NJ 07960 Shareholders will be admitted to the Annual Meeting beginning at 8:00 a.m. Eastern Daylight Time. If you wish to attend, please plan to arrive early since seating will be limited. Please see the inside back cover of the Proxy Statement for directions. PLEASE NOTE – YOU CANNOT VOTE BY RETURNING THIS NOTICE. To vote your shares you must vote online or request a paper copy of the proxy materials to receive a proxy card. If you wish to attend and vote at the meeting, please bring this notice with you. Proposals to be voted on at the meeting are listed below and include the Board of Directors’ recommendations. The Board of Directors recommends a vote “FOR” the following proposals: 1. Election of Directors: Dennis A. Ausiello, M. Anthony Burns, W. Don Cornwell, Frances D. Fergusson, William H. Gray, III, Helen H. Hobbs, Constance J. Horner, James M. Kilts, George A. Lorch, John P. Mascotte, Suzanne Nora Johnson, Ian C. Read, Stephen W. Sanger, Marc Tessier-Lavigne 2. Ratify the selection of KPMG LLP as independent registered public accounting firm for 2012 3. Advisory approval of executive compensation The Board of Directors recommends a vote “AGAINST” the following proposals: 4. Shareholder proposal regarding publication of political contributions 5. Shareholder proposal regarding action by written consent 6. Shareholder proposal regarding special shareholder meetings 7. Shareholder proposal regarding advisory vote on director pay If you plan to attend the Annual Meeting, please bring this admission ticket with you. Here’s how to order a copy of the proxy materials and select a future delivery preference: Paper copies: Current and future paper delivery requests can be submitted via the telephone, Internet or email options below. Email copies: Current and future email delivery requests must be submitted via the Internet following the instructions below. If you request an email copy of current materials you will receive an email with a link to the materials. PLEASE NOTE: You must use the number in the shaded bar on the reverse side when requesting a set of proxy materials. 3 Internet – Go to www.investorvote.com/PFE and follow the instructions to log in and order a copy of the current meeting materials and submit your preference for email or paper delivery of future meeting materials. 3 Telephone – Call at 1-866-641-4276 or 1-781-575-4581 for outside the USA, US territories and Canada and follow the instructions to log in and order a paper copy of the materials by mail for the current meeting. You can also submit a preference to receive paper copies for future meetings. 3 Email – Send email to investorvote@computershare.com with “Proxy Materials Pfizer Inc.” in the subject line. Include in the message your full name and address, plus the number located in the shaded bar on the reverse, and state in the email that you want a paper copy of the current meeting materials. You can also state your preference to receive paper copies for future meetings. To facilitate timely delivery, all requests for a paper copy of the proxy materials must be received by April 12, 2012. 002CS40011 01F7DJ